Designing an R&D Preparedness and Response Ecosystem for Potentially Pandemic Pathogens
It is mid-September 2020, and SARS-CoV-2 is thriving, continuing to spread wherever well-proven public health measures are poorly implemented. The United States is experiencing deaths equivalent to those that would have been caused by two World Trade Center attacks per week, and on September 11, 2020, the Institute for Health Metrics and Evaluation at the University of Washington predicted that the death count in the United States alone could exceed 415,000 by the end of the year (Institute for Health Metrics and Evaluation, 2020).
It is increasingly clear that full control over the pandemic will remain elusive without a safe and effective vaccine and the willingness of people to be immunized. While vaccines are being developed at unprecedented speed, the goal of “sequence to proof of concept” in four months is not sufficiently ambitious to stop a pandemic in its tracks. Moreover, economic and political realities that are being characterized as “vaccine nationalism” may delay access to a COVID-19 vaccine for half the world’s population.
This paper highlights issues for the Sabin-Aspen Vaccine Science & Policy Group to consider as members ponder ways to do better the next time. Undoubtedly, much of the solution will need to emanate from the political will and economic commitment of world leaders. But some can also come from the continued evolution of the research and development (R&D) ecosystem toward what we now characterize as an end-to-end R&D Preparedness and Response Ecosystem. Our views here build from a report we recently prepared at the request of the Global Preparedness Monitoring Board, based on a literature search, background interviews with 54 global leaders conducted during February through April 2020 as well as our prior experience in global health issues (Keusch & Lurie, 2020). They lead to three basic concepts:
- A good pandemic vaccine response must build on a strong, well-functioning, day-to-day system
- One can always start the work, but we can’t make up for lost time
- A vaccine response cannot depend on passing a tin cup in the middle of a pandemic
An evolved, end-to-end R&D Preparedness and Response Ecosystem must become the strong, well-functioning, day-to-day system. The challenge is how to get there as fast as possible.
Conceptualizing an Ecosystem for R&D
The concept of a health sector R&D ecosystem was initially driven by the pharmaceutical industry’s need to harvest basic discovery and more efficiently accelerate the drug development process by aligning a diverse set of stakeholders in partnerships that included both traditional competitive and collaborative R&D efforts (Pfizer, n.d.). This construct has evolved to include a host of public-private partnerships aimed at improving the efficiency of translational science (World Bank, n.d.).
However, an R&D ecosystem for pandemic preparedness and response must address additional uncertainties and challenges. Scientifically, it is focused on products whose characteristics and ultimate purpose may not yet be known and may be developed for a market that may never exist. The manner in which such products may be designed for use can only be predicted in the abstract and may be intended for a time that may never arrive. While the needs can be imagined, the specifics cannot be known, requiring a new way of thinking about existing evidence, assessment of probabilities and a willingness to investigate and invest before the relevance of the evidence is clear.
Scientific advancements developed through the ecosystem can only lead to an effective epidemic response if they can be acted upon quickly. That requires not only a body of relevant scientific information but also trained scientists, laboratories, development partners, trial networks, rapid funding and oversight, leadership and governance, all pre-positioned to act. Furthermore, effective continuity between preparedness R&D activities before an outbreak occurs and targeted R&D responses to the emergent pathogen afterward needs to be assured. The impact of the R&D process depends not only on the development of safe and effective products but also a means to manufacture them at a scale sufficient for pandemic needs and the mechanisms to finance and equitably distribute them wherever required.
There has been significant evolution of this ecosystem over the past decade, accelerated by the 2014-16 Ebola outbreak in West Africa. Notable progress can be found in the creation of global partnerships and mechanisms for new pre-pandemic or preparedness R&D, such as the development of One-Health approaches, the World Health Organization (WHO) R&D Blueprint, the Global Research Collaboration for Infectious Disease Preparedness (GloPID-R), the creation of the Coalition for Epidemic Preparedness Innovations (CEPI) and the prototype-pathogen approach that the National Institutes of Allergy and Infectious Diseases (NIAID) had taken to pandemic preparedness several years before the current outbreak (Marston et al., 2017).
In other words, over the course of a decade, the system has evolved from business as usual in pharmaceutical R&D to an enlarged R&D ecosystem, with industry involved in both competitive and collaborative research efforts, and then further to a new concept of an R&D preparedness ecosystem. That is where the world was on January 1, 2020, shortly after the WHO was informed of a disease outbreak in Wuhan, China, that was causing severe pneumonia and respiratory failure, with a high case fatality rate. That turned out to be a novel coronavirus.
The existing preparedness ecosystem allowed R&D to shift rapidly in response. The sequence of the new virus was posted on January 11, 2020, and two days later scientists at the Vaccine Research Center of the NIAID decided to evaluate the spike protein of the virus and prepare the RNA sequence for an mRNA vaccine candidate, a vaccine platform they had been developing for several years in collaboration with the biotech company Moderna (Dance, 2020). Within a few days, the Vaccine Research Center team had synthesized the protein and examined its 3D structure to identify the most likely antigens to target with a vaccine, determined how to stabilize the structure, and engaged with Moderna to create the specific mRNA vaccine and begin studying the vaccine’s ability to induce the host to synthesize the peptide and mount an immune response. Within six weeks, the first Phase 1 trials began.

Shortly before the sequence was posted, CEPI also began to mobilize its resources and partnerships to kickstart work with a variety of developers, including its existing vaccine R&D partners. With these and additional efforts from pharma and biotech, the number of vaccine development efforts focused on the new virus rapidly expanded, with a variety of approaches supported by different industry and academic groups and with various public sources of funding.
It is sometimes easy to overlook the fact that the R&D ecosystem in the United States has a number of fundamental differences from that of the rest of the world. As the response to COVID-19 — and before it H1N1 — illustrate, government funds support the bulk of the underlying science that goes into accelerated emergency vaccine development. For example, the NIAID and others support basic science, and the NIAID and the Centers for Disease Control and Prevention (CDC) support basic epidemiology; share virus specimens, animal models and laboratory resources; obtain the biological specimens needed to understand the immune response; and develop assays and diagnostics. Biomedical Advanced Research and Development Authority (BARDA) funding supports advanced product development (although emergency supplemental funds may be needed), as well as manufacturing at risk and at needed scale, and vaccine procurement for domestic needs. Finally, the CDC supports domestic vaccine distribution and a vaccination campaign.
As CEPI kicked off its vaccine development work, it rapidly confronted the fact that there were no pre-identified, go-to entities with the mandate, responsibility and funding to conduct the early enabling-science work — functions we take for granted in the United States. In other words, while the global scientific community is quite strong, there were no labs charged specifically with growing and sharing the virus with other labs and investigators, developing and supporting animal models, collecting and curating biological specimens, and so on. The ecosystem contained all these elements, but each depended on entrepreneurism and new funding to act. And, like a conductorless orchestra, each component played its part, often exceedingly well, but not always in a way to support an ideal tempo or harmony, or to eliminate needless repetition of movements. Despite these challenges, the extent of the global scientific collaboration has been staggering, and vaccine development has proceeded quickly both within the United States and, because CEPI had been established, outside it.
CEPI realized early that the requirements of the job would not be met once a successful candidate was developed. Yet no entity in the world had the mandate to support manufacturing the billions of vaccine doses needed in the face of a global crisis. Moreover, although industry has been heavily involved, it was not reasonable to expect the private sector to invest in manufacturing at risk and at scale without advance purchase commitments and financing to support the development and clinical trials of vaccine candidates. Similarly, there was no entity responsible for buying vaccine doses and distributing them globally and equitably in order to end the pandemic as quickly as possible.
Thus, the global ecosystem has had to adapt quickly. Global stakeholders came together to create the Access to COVID Tools Accelerator (ACT-Accelerator), which was launched on April 24, 2020, and encompassed diagnostics, therapeutics, vaccines, personal protective equipment and health systems strengthening. Within it, the COVAX pillar, co-led by CEPI, Gavi and the WHO, is an attempt to link vaccine development, procurement and delivery in an end-to-end fashion. The COVAX facility, run by Gavi, is envisioned as a purchasing agent for both self-financed and subsidized country purchases. At the time of this writing, however, the COVAX partners were still struggling to raise the needed funding to support manufacturing scale-up and scale-out, advanced purchase commitments sufficient to incentivize manufacturing at risk, and dose procurement, as well as support of delivery to low- and middle-income countries.
Meanwhile, high-income countries are engaging in multiple bilateral deals with manufacturers, threatening to drive up prices and compromise equitable global distribution. The lack of any system, let alone a strong day-to-day system, to ensure that vaccines are not only developed but also manufactured, distributed and delivered without delay should be clear to all. It is also evident that stopping the R&D ecosystem activities once a vaccine has been shown to be safe and effective will not address the full scope of the challenge.

The opportunity we have now is to develop a new paradigm — a unified, end-to-end R&D Preparedness and Response Ecosystem that begins with basic science and strong global disease surveillance and ends with vaccine administration sufficient to stop a pandemic. The question now turns from whether to invest in this to how to connect the pieces. That means identifying the effective ways to make connections at the same time that barriers are exposed and dismantled, overcome or bypassed. The challenges in manufacturing point to a part of the ecosystem that has continued to be neglected: innovation in manufacturing to simplify, speed up and de-risk the technical components of technology transfer and scale-out, as well as the manufacturing process itself. Early efforts through the WHO and PATH to do this for flu vaccine product formulation and sterile packaging (i.e., fill-finish) processes may serve as a model go (WHO, 2012).
We should note that a similar exercise has occurred in the development of therapeutic monoclonal antibodies. These are potentially of value to provide instantaneous passive immunity to a person exposed to a pathogen and to preclude infection in high-risk subjects, such as health care workers. They could also be useful therapeutically for individuals who are already infected and symptomatic or at an earlier stage to stop progression to clinical disease. A good example is the early decision by Regeneron to use its proven platform technology, which was successfully used to generate and then produce at scale humanized monoclonal antibodies to treat Ebola or prevent infection after exposure and is now being targeted at SARS-CoV-2. Shortly afterward, in early February, an existing collaboration with BARDA was extended to COVID-19 (Gallagher, 2020), and the NIAID subsequently co-funded clinical trials of the resulting product (National Institutes of Health, 2020). While such at-risk efforts are to be lauded, the risk exposure is of fundamental concern to pharma and biotech companies, and the R&D Preparedness and Response Ecosystem has to ensure that the funding to jumpstart development is in place.
What is the Nature of an R&D Preparedness and Response Ecosystem?
While we are of the mind that an end-to-end system is needed, starting with basic science and non-product-oriented research and stretching to the delivery of a vaccine to humans, that system is not necessarily linear. Indeed, to be responsive as fast as possible, it cannot be linear; many actions must be taken simultaneously.
This overarching ecosystem is made up of multiple smaller ecosystems, each functioning somewhat autonomously to identify and solve unique problems. Their activity levels may wax and wane as needs change, but each must always keep an eye on the others to determine when information sharing is going to be valuable or perhaps to actively overlap and partner, at least for some time. As a system of systems, the rules for connecting, partnering or operating in parallel are going to be fluid as demands evolve. They are also not predictable, at least not to the degree that traditional linear R&D in the private sector is required to be. That is surely a brake on ingenuity and invention, which makes the active management of the ecosystem something of a nightmare. Management is nonetheless essential, as we touch on shortly.
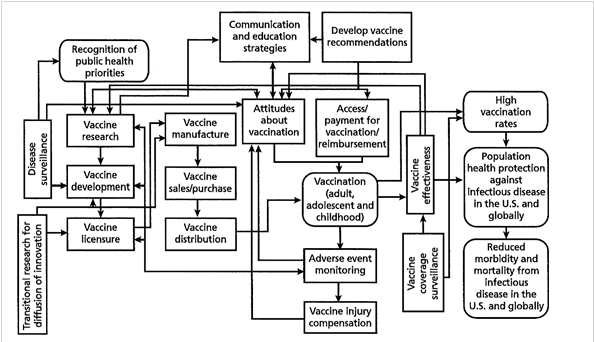
Because the ecosystem is clearly not two-dimensional, the usual organogram or process diagram drawn as a series of linked boxes (see Figure 1) cannot represent it, even if the various boxes can expand or contract over time as demands are altered. Because there is no way to predict where any of the parts are going to be in the future, the analogy becomes, in effect, more and more like quantum mechanics. It is also subject to Heisenberg’s uncertainty principle, which posits that the momentum and position of a particle (analogous to a mini-system) cannot both be precisely determined at the same time, even in theory. Without the ability to use statistical methods to assess where these R&D particles are and how energized they are — because they cannot be clearly seen — how can they be managed?
Figure 1. Overview of the vaccine and immunization enterprise
Source: Ringel et al., 2009.
The other analogy, mentioned above, is that of a conductorless orchestra composed of expert sections, including strings, brass, percussion and so on, preferably each with its own concertmaster. They are all in an empty auditorium and, depending on their orientation, may not see one another, a setup that becomes further complicated if the sections decide to move about (see Figure 2). We think there is value in coming to grips with these dynamic challenges in order to better align the parts and improve the outputs and the speed with which they can be developed. In this context, the principles of system dynamics may be of particular value.
The System Dynamics Society defines system dynamics as “a computer-aided approach to policy analysis and design. It applies to dynamic problems arising in complex social, managerial, economic, or ecological systems — literally any dynamic systems characterized by interdependence, mutual interaction, information feedback, and circular causality” (Systems Dynamic Society, n.d.).
The R&D Preparedness and Response Ecosystem has those fundamental characteristics: interdependence, mutual interaction, information feedback and circular causality.
The challenge is to understand the parts and kinetics of the new ecosystem and how to guide the components to achieve the desired outcomes. Taking a system dynamics approach to this understanding might help elucidate a better governance approach for the preparedness-response ecosystem than we are able to propose here.
One of the planners of the Sabin-Aspen Vaccine Science & Policy Group asked whether the vaccine R&D ecosystem is a “wicked problem.”
The question refers to the work of design theorists Horst Rittel and Melvin Webber, who used the term to characterize the complexities and challenges of describing social policy problems and planning solutions (Rittel & Webber, 1973). Compared to “tame” problems in mathematics or chess, “the wicked problems of planning lack clarity in both their aims and solutions … a challenge of articulation and internal logic, [and] they are subject to real-world constraints that prevent multiple and risk-free attempts at solving.”
Rittel and Webber identify 10 important characteristics of wicked problems (see Table 1), which are similar to the challenges that system dynamics modeling attempts to solve. Importantly, the tenth characteristic states that planners — those who present solutions to these problems — have no right to be wrong. Unlike mathematicians, “planners are liable for the consequences of the solutions they generate; the effects can matter a great deal to the people who are touched by those actions.”
Table 1. Characteristics of wicked problems
- They do not have a definitive formulation.
- They do not have a “stopping rule” and lack an inherent logic that signals when they are solved.
- Their solutions are not true or false, only good or bad.
- There is no way to test the solution to a wicked problem.
- They cannot be studied through trial and error because their solutions are irreversible, so, as Rittel and Webber put it, “every trial counts.”
- There is no end to the number of solutions or approaches to a wicked problem.
- All wicked problems are essentially unique.
- Wicked problems can always be described as the symptom of other problems.
- The way a wicked problem is described determines its possible solutions.
- Planners, that is those who present solutions to these problems, have no right to be wrong. Unlike mathematicians, “planners are liable for the consequences of the solutions they generate; the effects can matter a great deal to the people who are touched by those actions.”
Source: Stony Brook University, n.d.
To approach the management and governance of an R&D Preparedness and Response Ecosystem as a wicked problem may require broad-based and collaborative reasoning and help from system dynamics modelers to achieve focused future solutions. It will also need to evolve a management and governance structure that allows it to function freely and yet reins it in sufficiently to concentrate on practical solutions. Equally essential is how to organize and provide the required financial resources to make it work in a responsible manner. Even if not wicked, this is a tricky task.
What Conditions Will Energize an End-to-End Preparedness and Response Ecosystem for R&D
Unless we believe the low-cost Albert Einstein thought-experiment approach will work here, an R&D Preparedness and Response Ecosystem will need substantial funding to meet its goals both before an outbreak and in response to one. To attract the necessary resources, it is essential to address the reluctance to invest before a pathogen proves its ability to cause outbreaks, epidemics or pandemics. While investing in pathogen-agnostic antigen expression platforms is a good start, the prime barrier to early investment is that preparedness R&D research is scientifically focused on products whose characteristics, ultimate purpose and need are not yet known. As we commented earlier, the needs can be imagined, but the specifics are unknowable, requiring a new way of thinking about virulence and host-shifting capacity, assessment of the probability of future emergence and a willingness to investigate and invest before relevance is certain.
While continued early investments are key, situating those investments and capabilities as pre-positioned and globally distributed resources to respond to an emerging outbreak is critical. We propose that hubs for the core enabling scientific activities described in Table 2 be identified and positioned now. In most cases, there should be at least one hub per continent, both to promote equity in accessing scientific resources and because a novel pathogen can arise anywhere, although there are known hotspots for emergence. Standing capacity for this international research consortium could be collaboratively funded by scientific agencies around the globe.
| Enabling Science Activities | What Happened During COVID-19 Pandemic | Proposed R&D Preparedness Actions |
|---|---|---|
| Posting and curating gene sequences throughout the pandemic. | Virus was sequenced privately weeks before being posted publicly and identified as a novel coronavirus. After initial posting on several sites, GISAID became central because it is the de facto go-to site for most of the scientific community. Largely maintained by volunteer scientists, GISAID received many more sequences than it could handle, and its funding was insufficient to function as needed. | Expand GISAID agreements to cover all viruses or establish a new and professional repository. Ensure adequate funding to curate pathogen sequences and maintain quality control. Consider a global treaty to reinforce need and commitment to early sequence posting and sample sharing for pandemic response. |
| Receiving, growing and sharing virus samples with laboratories around the world. | China did not make virus samples available. Once COVID-19 cases surfaced in other countries, several labs voluntarily grew virus and shared samples through their networks. However, no entity or network had a mandate or the necessary financial support for this. Nor was there an organized system with rules to prioritize requests for samples. | Pre-position and fund a global network of laboratories, at least one per continent, with the mandate and capability of receiving, growing and safely shipping virus specimens to investigators and product developers. Define the mechanism to vet and prioritize requesting entities. |
| Collecting, curating and disseminating human biological reference material. | Initially, collection of materials was opportunistic and unstandardized, and clinical samples were not necessarily appropriately stored. Validation panels for diagnostic tests are still in very short supply and often not standardized. No entities have a clear mandate or funding to take on these tasks. The National Institute for Biological Standards and Control (NIBSC), a WHO collaborating center, was funded by CEPI to develop antibody standards, but work was slow to start because of time lags in specimen collection. | Pre-position and fund a global network of investigators and laboratories, at least one per continent, with the mandate and capability of ethically collecting, managing and sharing specimens and curating standardized specimen collections. |
| Developing, validating and sharing animal models that replicate human disease progression, manifestations and pathology. Availability of containment laboratory and testing resources operating at international good laboratory practice standards or, under the Food and Drug Administration animal rule, well-controlled models and well-documented data. | Lack of immediate funding slowed animal model development and testing in labs that had available containment laboratory capabilities. Existing labs are at capacity and have struggled to keep up with demand. | Pre-position and fund a global network of laboratories and investigators, at least one per continent, with biosafety levels 3 and 4 capabilities. CEPI has recently funded such a network, but for vaccine development only. Invest in new technologies to reduce reliance on animal testing (e.g., organ-on-a-chip), and develop genetically engineered humanized mouse models tailored to the pathogen and human immune response. |
| Defining the basic epidemiology of the disease, with a focus on key knowledge gaps related to outbreak control and needs for product development. | Data collection was haphazard, poorly standardized and often inappropriately analyzed and published in pre-peer review platforms that were widely read and sometimes promoted unproven and potentially dangerous interventions. Modeling the course of an outbreak can only be as good as the quality of the data used in the model. | Strengthen International Health Regulations capacity in all countries, with capable and resourced national public health institutes closely linked to regional and global partners, including the active and proactive involvement of WHO and partners. |
| Pre-position clinical trial networks with established adaptable protocols, ethical review standards and multi-site comparable and common quality-control methods in place for therapeutics and vaccines. | Numerous poorly designed, inadequately powered therapeutic studies with uninterpretable data. UK-based RECOVERY trial, launched in March 2020 and supported by the UK National Health Service (NHS) and others, a randomized controlled clinical trial to compare commonly available drugs as potential therapeutics across the whole NHS system. It rapidly recruited patients and generated conclusions. In April 2020 NIH launched its version, the Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership, for adaptive trials of promising treatments as well as vaccines. In March 2020 the WHO launched Solidarity, a multi-country adaptive therapeutics trial that has now produced interim results. No multi-arm studies for vaccines, as of yet. | Establish standing infrastructure and financing for randomized controlled multi-arm adaptive clinical trials for therapeutics and vaccines, with an objective, expert and flexible governance mechanism to coordinate protocol design, scientific and ethical review, selection of products to trial and patient inclusion criteria, outcome variables, safety monitoring, analysis and reporting. |
| Manufacturing scale-up and innovation. | Initially, no clear funding source for use or development of manufacturing capacity. High-income countries are buying up products in advance of approval for their own use (“vaccine nationalism”) with simultaneous creation of global collaborative mechanisms to ensure products are available and subsidized for at-risk low- and middle-income countries, based on need and impact. | Pre-position and fund global network of manufacturing facilities for diagnostics, therapeutics and vaccines that can surge quickly to manufacture quality products in sufficient quantities. Promote manufacturing innovation to produce at required speed and scale faster. Develop new global mechanisms for product accession and fair distribution procedures, based on public health need and high potential to impact outbreak control. |
Source: Keusch and Lurie analysis.
An operational expert research coordinating entity, potentially evolving from GloPID-R but with broader mandates for co-funding and the ability to move quickly, could be responsible for identifying and pre-positioning such implementing partnerships, encouraging equity of opportunity and recompeting and reevaluating the partnerships every five to 10 years. To be successful, pooled funding would also need to be pre-identified and available for release in a matter of weeks.

The World Bank’s 2018 report, Money and Microbes (World Bank, 2018), suggested that a multi-donor fund held at the bank could support such activities, but other mechanisms may be similarly satisfactory. A key preparedness activity of the coordinating entity might be an annual, global scientific preparedness drill. Its purpose would be to strengthen global collaboration and capacity where outbreaks often originate and further the WHO’s unique role in establishing norms for global behavior (e.g., data sharing, material transfer agreements, common protocols and ethics reviews) that can be leveraged in a pandemic.
When the full economic costs of the COVID-19 pandemic are calculated, it is certain they will far exceed the cumulative investment needed to put this R&D Preparedness and Response Ecosystem in place. Such a mechanism can preclude the enormous and ongoing loss of life and prevent the disruption of social, cultural, political and economic stability around the world. A major concern going forward is how to keep the sponsors involved for the long term; the examples of the U.S. government unilaterally exiting from multiple global compacts, including its decision to quit the WHO, are — in our minds — shortsighted, reprehensive and xenophobic.
Advancing global R&D preparedness with these early activities will also require a framework and threshold for activation (National Academy of Medicine, 2016). Not all outbreaks will become pandemics, and for this ambitious venture to be effective, there will be occasions when the global system and rapid expenditure mechanisms are activated and then quickly wound down because the outbreak is effectively contained, with minimal need for new countermeasures. Such a system is similar to the CDC’s Influenza Risk Assessment Tool, which evaluates the pandemic potential of novel influenza strains to provide guidance on how far into the vaccine development process the U.S. government should go.
The philosophy that one can always start the development process and then take an off ramp if the risk level is low enough also recognizes that it is impossible to make up for lost time. In this sense, rapid activation, a clear global good, should be viewed as a “cost of preparedness,” akin to paying an insurance premium, but one that also builds capacity and provides the opportunity to practice for future events. Response to real-world events, when they occur, should be supplemented by research-response exercises to continually identify and overcome barriers. To be successful, a research-response fund would need a “no-regrets” annual budget to ensure that resources are always available and can be rapidly released, ideally within a week or two following a request. A sound objective mechanism for coordinating this effort is essential.
At the other end of the ecosystem, a different kind of financing is critical — a readily available reserve fund sufficient to support manufacturing at risk, procurement, distribution and administration of vaccine doses when a pandemic occurs. Estimates from the International Monetary Fund are that the global economy will take a $12 trillion loss in 2020-21 due to COVID-19; accelerating vaccine availability by a single month could save as much as $500 billion (Gopinath, 2020). Clearly, a reserve fund equivalent to even a week’s losses of this magnitude would be a sound investment. The challenge now is for global leaders to muster the political will to mobilize the resources and design a governance structure for its use.
Meanwhile, vaccine development funders can continue to innovate in new technologies, from novel ways to stimulate B-cells to produce antibodies through next-generation, pathogen-agnostic vaccine development programs and innovations that will enable speedy, safe, light-footprint manufacturing capacity on each continent. Figuring out how best to conduct the remainder of the vaccine development symphony remains a tricky problem, if not a wicked one. New tools, such as system dynamics modeling, can meaningfully contribute to the solutions.
Central Relevance of Peripheral Issues
Developing vaccines is a hardcore scientific process, to which formulation know-how, at times empiric rather than rational, is essential. It is not the place for amateurs to take on the highly critical responsibility for governance of the R&D Preparedness and Response Ecosystem of vaccines for future pandemic pathogens. Its leadership requires basic scientists, formulation experience, translational research expertise and the ability to obtain licensure. Delivering vaccines to those who need them is another process issue, peripheral to vaccine development but essential to ensure that they are used at the population level. For new pandemic threats like SARS-CoV-2, that means at least 70 percent of the world’s population, or whatever the threshold for herd immunity proves to be. Vaccine developers do not organize vaccine delivery, just as vaccine delivery experts do not develop them. But the effort and expense of development is futile if an effective vaccine sits on a warehouse shelf. This is what we mean by a centrally relevant peripheral issue.
All of that raises the question of the essential and proper role of the WHO in governance. The WHO is the only global public health institution for everybody — hence its name — and it serves a number of essential roles. One of the most important is to be the voice of those who are otherwise without one — people living in low- and lower-middle-income countries that often have poor health care systems and limited capacity to address population-level issues. The WHO must advocate for their concerns to the powerful and wealthy, who by and large dominate the scientific enterprise. The WHO is also the critical definer of normative standards for health and the defender of inclusive policies so that all at risk can benefit from medical advances, such as a safe and effective vaccine for COVID-19. As the race to develop this essential tool escalates, and vaccine nationalism rears up, the WHO is one of the few global entities that can convince and organize nations to take the moral high ground and promote equitable access. Again, this is an issue centrally relevant and yet peripheral to vaccine development.
Also centrally relevant, yet peripheral, is the availability of sensitive, specific, simple and speedy diagnostic tests. The efficacy of a vaccine cannot be tested if the pathogen cannot be identified among participants of rigorous controlled clinical trials who become ill. It is not the job of the vaccine developer to create these diagnostics, but it surely is necessary to their task.
The list can be extended to items such as personal protective equipment for health care workers and researchers caring for those potentially infected with the pandemic pathogen as part of clinical research and trials. As we have seen during COVID-19, the need extends to larger numbers of people doing work that may expose them to the virus, from grocery store clerks and delivery services to police and fire department personnel. Again, this is centrally relevant, but peripheral, to vaccine development.
We end this exercise in informed freethinking at this point. As the Sabin-Aspen Vaccine Science & Policy Group examines opportunities to advance the development of vaccines for pandemic pathogens like SARS-CoV-2 today, and others unknown tomorrow, in the most creative and hopefully audacious way possible, there are many relatively pedestrian issues to consider. We hope the group will advocate for the necessary support to build the broader end-to-end ecosystem we envision and believe is desperately needed. Equally essential is advocating for investments in high-risk/high-payoff new approaches to vaccine development and global funding mechanisms protected from precipitous failures due to sudden political shifts of key donor nations. Above all, having pulled out all the stops to achieve vaccine success, there must be a commitment to equitable access to a pandemic vaccine across the globe, regardless of a nation’s ability to pay for it.
Nicole Lurie is currently strategic advisor to the CEO of the Coalition for Epidemic Preparedness Initiatives (CEPI) and a senior lecturer at Harvard Medical School. She previously served as assistant secretary for preparedness and response at the U.S. Department of Health and Human Services, leading the Department’s response to infectious diseases, natural and manmade disasters, and other public health emergencies. Prior to federal service, she was the Paul O’Neill Professor of Policy Analysis at RAND, where she started and led the public health preparedness program. Lurie’s research has focused on access to and quality of care, health system redesign, equity, mental health, public health, and preparedness. A member of the National Academy of Medicine, she continues to practice clinical medicine in a community clinic in Washington, D.C. Lurie received her doctor of medicine from the University of Pennsylvania and completed her residency and public health training at University of California, Los Angeles.
Gerald T. Keusch is a board-certified internist and infectious diseases specialist, and an academic physician-scientist who has served on the medical school faculty at Mt. Sinai, Tufts University and Boston University. After serving as chief of infectious diseases at Tufts from 1986 to 1998, he became associate director for international research and director of the Fogarty International Center at the National Institutes of Health. At Boston University, he is associate provost for global health and associate director of the National Emerging Infectious Diseases Laboratory maximum containment facility. Author of over 300 research publications and book chapters, he has received multiple awards for his contributions to medicine and science, including the Avery, Fleming and Finland Lecture awards from the Infectious Diseases Society of America. Keusch was elected to the National Academy of Medicine (formerly the Institute of Medicine) in 2002 and has co-chaired three recent reports on emerging infectious diseases. He received his medical degree at Harvard Medical School.
References
Dance, A. (2020, July 21). Coronavirus vaccines get a biotech boost: Advances in technology are accelerating the search for drugs to arm the immune system against SARS-CoV-2. Nature. https://www.nature.com/articles/d41586-020-02154-2
Gallagher, G. M. (2020, February 6). HHS and Regeneron partner on coronavirus antibody treatment. Contagion Live. https://www.contagionlive.com/news/hhs-and-regeneron-partner-on-coronavirus-antibody-treatment
Gopinath, G. (2020, June 24). Reopening from the great lockdown: Uneven and uncertain recovery. IMFBlog. https://blogs.imf.org/2020/06/24/reopening-from-the-great-lockdown-uneven-and-uncertain-recovery/
Institute for Health Metrics and Evaluation. (2020). Results briefing: United States of America. Model updates, September 11. http://www.healthdata.org/sites/default/files/files/Projects/COVID/briefing_US_091120.pdf
Keusch, G. T., & Lurie, N. (2020). The R&D Preparedness Ecosystem: Preparedness for health emergencies. Global Preparedness Monitoring Board. https://apps.who.int/gpmb/assets/thematic_papers_2020/tp_2020_5.pdf
Marston, H. D., Paules, C. I., & Fauci, A. S. (2017). The critical role of biomedical research in pandemic preparedness. JAMA, 318(18), 1757–1758. https://doi.org/10.1001/jama.2017.15033
National Academy of Medicine. (2016). The neglected dimension of global security: A framework to counter infectious disease crises. The National Academies Press. https://doi.org/10.17226/21891
National Institutes of Health. (2020, August 10). Clinical trials of monoclonal antibodies to prevent COVID-19 now enrolling [Press release]. https://www.nih.gov/news-events/news-releases/clinical-trials-monoclonal-antibodies-prevent-covid-19-now-enrolling
Pfizer. (n.d.). R&D ecosystem. Retrieved October 30, 2020, from https://www.pfizer.com/research/rd_partnering/rd_ecosystem
Ringel, J. S., Adelson, M., Harris, K. M., Khodyakov, D., & Lurie, N. (2009). Improving the impact and effectiveness of the National Vaccine Advisory Committee. RAND Corporation. https://www.rand.org/pubs/technical_reports/TR752.html
Rittel, H. W., & Webber, M. M. (1973). Dilemmas in a general theory of planning. Policy Sciences, 4(2), 155–169. https://doi.org/10.1007/BF01405730
Stony Brook University. (n.d.). What’s a wicked problem? Retrieved October 30, 2020, from https://www.stonybrook.edu/commcms/wicked-problem/about/What-is-a-wicked-problem
System Dynamics Society. (n.d.). What is System Dynamics? Retrieved October 30, 2020, from https://www.systemdynamics.org/what-is-system-dynamics
Weintraub, R., Bitton, A., & Rosenberg, M. L. (2020, May 22). The danger of vaccine nationalism. Harvard Business Review. https://hbr.org/2020/05/the-danger-of-vaccine-nationalism
World Bank. (2018). Money and microbes: Strengthening clinical research capacity to prevent epidemics. http://documents1.worldbank.org/curated/en/120551526675250202/pdf/126338-REVISED-27231-IVTF-Report-reduced.pdf
World Bank. (n.d.). Public-private partnerships. Retrieved October 30, 2020, from https://www.worldbank.org/en/topic/publicprivatepartnerships
World Health Organization. (2012). Report of the Second WHO Consultation on the Global Action Plan for Influenza Vaccines (GAP). https://apps.who.int/iris/bitstream/handle/10665/44794/9789241564410_eng.pdf
While we are of the mind that an end-to-end system is needed, starting with basic science and non-product-oriented research and stretching to the delivery of a vaccine to humans, that system is not necessarily linear. Indeed, to be responsive as fast as possible, it cannot be linear; many actions must be taken simultaneously.