POWERING VACCINE R&D:
OPPORTUNITIES FOR TRANSFORMATION
EXECUTIVE SUMMARY
 When the Sabin-Aspen Vaccine Science & Policy Group decided in 2019 to consider ways to improve the research and development (R&D) component of the vaccine/vaccination ecosystem, SARS-CoV-2 (formally, severe acute respiratory syndrome coronavirus 2) was an unknown virus, and the word COVID-19, the disease it causes, had yet to be coined. While the subsequent pandemic made vaccine R&D appear to have been a prescient choice for the group’s annual meeting and report, we had initially chosen the topic to expand on our first report, Accelerating the Development of a Universal Influenza Vaccine, which focused on a single vaccine (Sabin-Aspen Vaccine Science & Policy Group, 2019).
When the Sabin-Aspen Vaccine Science & Policy Group decided in 2019 to consider ways to improve the research and development (R&D) component of the vaccine/vaccination ecosystem, SARS-CoV-2 (formally, severe acute respiratory syndrome coronavirus 2) was an unknown virus, and the word COVID-19, the disease it causes, had yet to be coined. While the subsequent pandemic made vaccine R&D appear to have been a prescient choice for the group’s annual meeting and report, we had initially chosen the topic to expand on our first report, Accelerating the Development of a Universal Influenza Vaccine, which focused on a single vaccine (Sabin-Aspen Vaccine Science & Policy Group, 2019).
As SARS-CoV-2 spread, the group retooled to ensure that we learned as much as possible from the unfolding pandemic, the historic pace of the response and the revolutionary technologies that were put to such swift use to combat it. Recognizing the imperative to be better prepared for the next such crisis, we also saw a crucial need to draw on experiences with prior and ongoing vaccine R&D for emerging diseases such as Ebola and Zika; to consider perennial challenges, such as influenza, malaria, tuberculosis (TB) and human immunodeficiency virus (HIV); and to ponder needed improvements on some existing vaccines, such as pertussis. There are both similarities and significant differences in the nature of these threats and how they need to be addressed, but each has something to teach us about the strengths and flaws in the current R&D enterprise — and the path forward.
CALL TO ACTION
As we learn the lessons from the past, we expect to see opportunities to overhaul current practices in time for the next pandemic, whenever it may strike; to make strides against the many endemic diseases that remain without adequate vaccines; and to accelerate the development of next-generation vaccines that improve performance or facilitate access and demand. All of that responds to the warning that infectious diseases have repeatedly sounded: do not neglect us.
With novel scientific tools and technologies opening new pathways for vaccines, and a recognition that equity in the R&D process must be as much of a priority as equity at the last mile, our work is very much of the moment. A brief look back also lends perspective to the ultimate purpose of the vaccine enterprise. Vaccines are one of the top 10 public health achievements of the 20th century, according to the U.S. Centers for Disease Control and Prevention (Centers for Disease Control and Prevention, 1999), and what the World Health Organization (WHO) calls a “global health and development success story” (WHO, n.d.-d). A critical bulwark against infections, the value of vaccines is never clearer to people or governments than during pandemics, as COVID-19 clearly demonstrates.

On another hopeful note, pandemics can be powerful stimuli for innovation. U.S. government investments in vaccines and other infectious disease research following the influenza pandemic of 1918-19 contributed to a twenty-twofold reduction in deaths from diseases among U.S. troops in World War II compared to World War I (Bush, 1945). The military-funded research also laid the groundwork for the postwar development of vaccines against the viruses that cause yellow fever, polio, measles, rubella, and hepatitis A and B (Duffy, 1992, pp. 270–279). Efforts by the WHO and other global institutions to ensure routine vaccination around the world helped to end smallpox, nearly eradicate polio and avoid more than 2 million to 3 million deaths each year from other vaccine-preventable illnesses (WHO, 2020b).
Increasingly, deploying vaccines against infectious diseases faces a new challenge — vaccination hesitancy, reflecting a breakdown in trust that has led to delayed vaccination or the refusal to receive them. The urgency of research into this last mile challenge — since vaccinations, not vaccines, prevent disease — was also a focus for the group, which spent a year of study before issuing its 2020 report, Meeting the Challenge of Vaccination Hesitancy (Sabin-Aspen Vaccine Science & Policy Group, 2020). Our return to R&D in this report reflects our continuing commitment to the end-to-end thinking that we believe is key to progress.
Vaccines and vaccinations are the two subsystems of a complex ecosystem, each with its own set of characteristics. The vaccine subsystem starts with the basic science that makes it possible for R&D processes to move forward and continues along a pathway that propels a product from the lab to approval for clinical use, commercial-scale manufacturing, and distribution, as well as the policies that govern these practices. Vaccination is the process of actually getting vaccines into human bodies, including the delivery and administration of the product, the willingness of individuals to be inoculated and the programs and policies that facilitate these steps.

The group’s decision to focus on R&D in 2020 reflected, in part, broad concerns about the declining number of vaccine developers (Danzon & Pereira, 2005; Wilsdon et al., 2020) and the structural failures that have slowed the arrival of new and improved vaccines. But those challenges stand beside remarkable accomplishments in recent years, including the new platforms that have enabled the rapid response to SARS-CoV-2. The arrival of several authorized vaccines less than a year after the virus was identified in December 2019 demonstrates the speed that is possible when we galvanize and align scientific capabilities with the necessary policymaking and financial resources. Timely data sharing, “real-time” publication and heightened collaboration across disciplines, sectors and borders were also essential contributors to the unprecedented pace of development.
The question is how those and other key elements of the accelerated process can be assembled, solidified, improved and applied to produce other needed vaccines — and then institutionalized so they are pandemic-ready. Malaria, TB and HIV remain among the top 10 causes of death in low-income countries (WHO, 2020c). And numerous other endemic infections, including Ebola, influenza, Middle East Respiratory Syndrome (MERS), Nipah virus and Zika, demand attention because they also pose significant risks to local populations and could seed future pandemics. While not all of the emergency measures used for SARS-CoV-2 need to or should be employed in every case, attention to restructuring R&D to produce essential vaccines for the infectious diseases we already recognize, and for those yet to emerge or be discovered, is imperative.
In that spirit, five key themes shaped the deliberations of the group:
- Developing and having timely access to safe and effective vaccines for all people is a moral imperative
- The devastation wrought by COVID-19 demands that we revisit and restructure the vaccine R&D enterprise
- Scientific breakthroughs and new technology expand ideas of what is possible
- A top-down, bottom-up approach, guided by coordinated leadership at all levels, advances vaccine R&D most effectively
- Public confidence in the R&D process is imperative to the ultimate goal of reducing the toll of vaccine-preventable diseases
FIVE BIG IDEAS TO POWER VACCINE R&D
The Sabin-Aspen Vaccine Science & Policy Group developed a package of five big ideas, summarized below, that we believe will engender a more efficient and responsive approach to vaccine R&D. The context for these ideas is detailed in the report that follows, which concludes by elaborating on each of them.
Define leadership roles, responsibilities and mechanisms of accountability to prepare for the R&D demands that surface in a pandemic. The global organizations that now have core roles to play in vaccine R&D need to map out real-time vaccine preparedness and response strategies so that they are ready to act as infectious diseases emerge or spread. Their leaders should convene in a structured setting to develop advance plans designed to surface key issues that need to be addressed and to make decisions about who will be responsible for taking them on, recognizing the imperatives of equity, clear communication and adequate capacity. A truly global consensus on how to move forward will require a multicentric leadership model that engages partners not only in the United States and Europe but also in China, Brazil, India and the African nations.
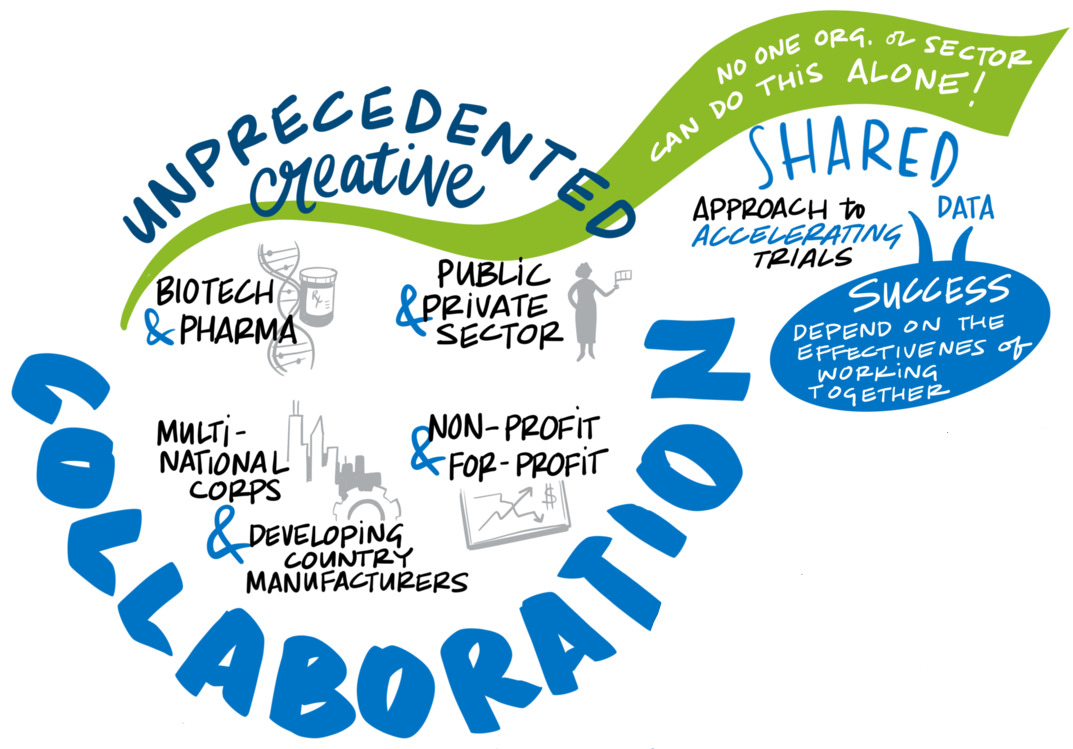
Propel a transdisciplinary research effort built around partnerships to expand and advance vaccine science. Nothing in recent times has showcased the payoff of basic science investment as much as vaccines to curb COVID-19. The vaccines are also a testament to the power of partnerships to break down institutional and competitive barriers to scientific collaboration. Such success can only become routine with intentional efforts to bring people together across fields in an environment designed to stimulate creative problem-solving and drive novel research and transformational science. To promote convergence, the group recommends support for a research infrastructure that creates opportunities for novel approaches and risk-taking; leverages lessons from adjacent scientific areas, ranging from the chemistry and physics of vaccine formulation to the immunologic basis of protection; and creates two-way learning opportunities between research focused on pandemics and on long-standing endemic diseases.
Reimagine clinical trials. Clinical trial design is ripe for more efficient and nimbler approaches. From the earliest design stage, clinical trials should consider the programmatic and policy questions likely to arise following vaccine authorization or approval. Equity and efficiency must also be prime drivers, with lower- and middle-income countries as full partners in clinical trial development at every stage. Bringing together large datasets and analyses of clinical and laboratory information on infected and vaccinated individuals may make it possible to identify immune correlates of protection, allowing regulatory approval to be based on smaller and faster trials. For example, the use of master protocols can accelerate trials and make findings easier to compare. Global networks of clinical trial sites and adaptive trial designs can speed data-gathering and postmarketing surveillance; a genuine commitment to advancing vaccine safety science is also key.
Restructure regulatory science to reflect advances in vaccine R&D. Transformative vaccine discoveries and technology must be accompanied by equally transformative approaches to regulatory science and process. Without compromising safety and efficacy, regulatory innovations should be pursued to streamline preclinical evaluation; make vaccine trials faster, nimbler and more cost-effective; and enhance product scale-up and manufacturing to improve global access. Harmonizing global regulatory standards and developing training opportunities, technical assistance and resource mobilization are key to strengthening and extending regulatory capacity, especially in lower- and middle-income countries.
Importantly, some changes may apply only to vaccines in emergency situations. For example, it may be appropriate to consider process improvements in the U.S. Food and Drug Administration (FDA)’s Emergency Use Authorization (FDA, 2021), the rolling reviews permitted by the European Medicines Agency to assess data as they become available (European Commission, 2020), and the WHO’s Emergency Use Listing procedure (WHO, n.d.-b). Other changes will apply across the board.
Position vaccines as a public good and align incentives so that benefits accrue to all sectors of society. Building on the recognition that the public has an interest in safe and effective vaccines and that companies expect to be rewarded for producing them, the incentives that drive action need to be examined in order to align them more closely. Agreeing on the research agenda is a front-and-center goal so that manufacturers, researchers and other core partners can be incentivized to pursue vaccine R&D that takes aim at the greatest threats to the global community. There should also be a strong push to develop or maintain policies and practices that promote information sharing. To guide alignment, criteria are needed to help determine which incentives should be offered and under what circumstances.
Primer on the R&D Component of the Vaccine/Vaccination Ecosystem
Vaccine R&D is the starting point for successfully generating any new vaccine, which traditionally has taken years or even decades to complete because of the rigors of the testing process and the complexity of regulatory approval. Many vaccines never get much beyond the conceptual stage, while others fail somewhere further along the development pathway (Dumonteil et al., 2019).
A Web of Activities
Like natural ecosystems, the R&D component of the vaccine/vaccination ecosystem is a complex and dynamic network with many distinct parts:
- Discovery and scientific research take place in the laboratory and generate the pharmaceutical product to be studied
- During preclinical research, candidate vaccines are developed and
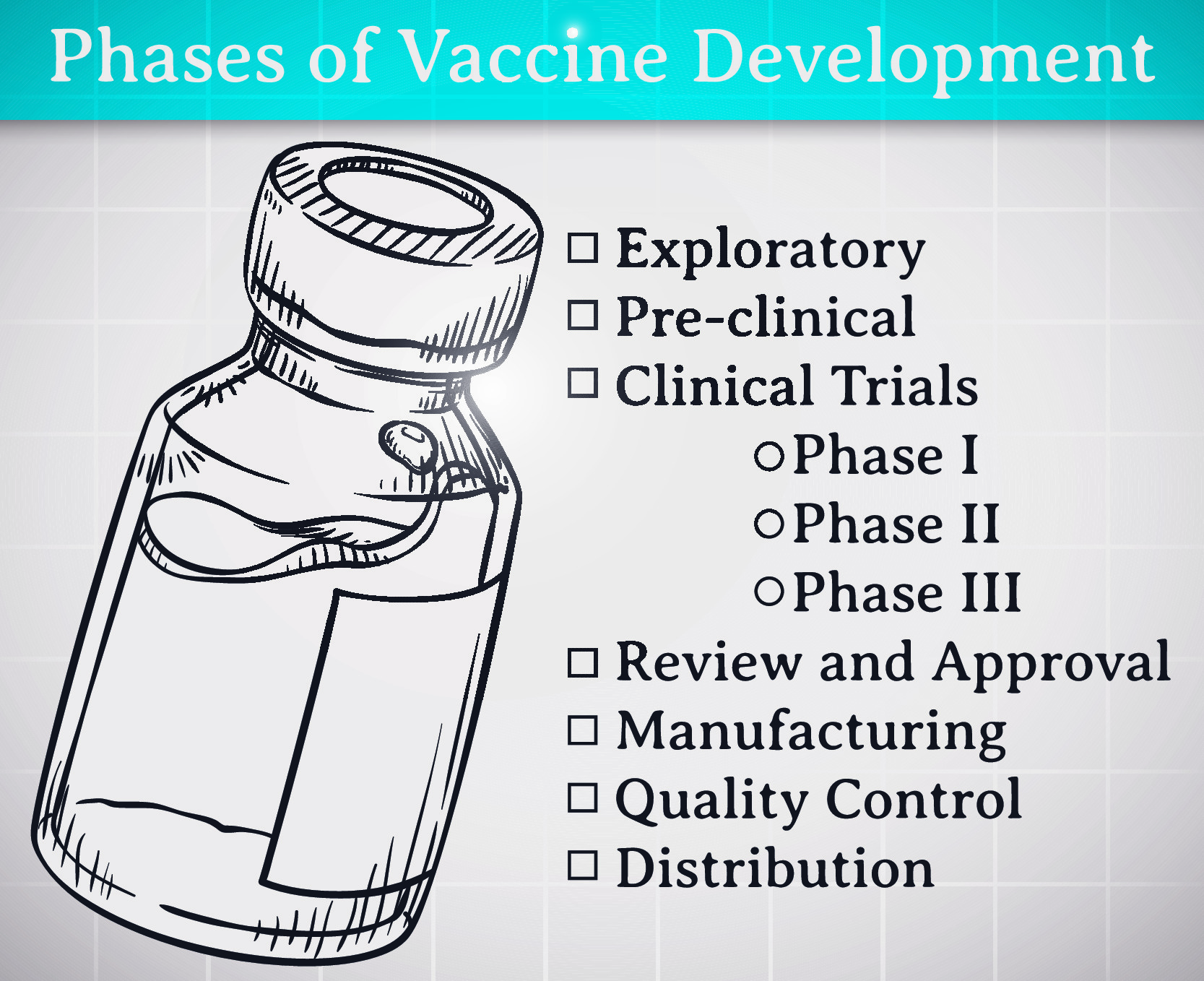 tested in animals to assess basic safety and immunogenicity before they
tested in animals to assess basic safety and immunogenicity before they
are evaluated in human beings - Three phases of clinical testing establish vaccine dosage and scheduling regimen and gather safety, immunogenicity and efficacy data in increasingly larger populations
- Scale-up and large-scale manufacturing consistently producing the needed volume of vaccines
- Regulatory submission and approval allows the product to come to market
These processes vary considerably by country and are intended to ensure that a product meets safety and efficacy standards before it is released for public use. 1This consensus framework definition was circulated to the Sabin-Aspen Vaccine Science & Policy Group to guide its discussions, but it is not an officially or universally recognized definition.

Note that the entire vaccine/vaccination ecosystem has other components that need to work together seamlessly for optimal performance. To name just a few, more effective global surveillance and genomic sequencing capacity are priorities to ensure rapid response before a pathogen engenders a pandemic. Further along the continuum, distributed manufacturing capacity, especially for newer-platform vaccines, is essential to ensure global availability. Other value-added opportunities include improved storage strategies and better technologies for deployment and administration — the last inch of the last mile. The Sabin-Aspen Vaccine Science & Policy Group supports heightened attention to these and other issues, but they are not the subject of this report.
The three phases of clinical testing that are generally required for regulatory approval have historically occurred sequentially, but the accelerated development of COVID-19 vaccines has shown that these processes can operate in parallel without sacrificing safety and immunogenicity assessments when the need is urgent.
- Phase 1 tests a promising vaccine in a small number of healthy volunteers to assess safety and immunogenicity
- Phase 2 engages several hundred volunteers and expands the numbers for safety signals while beginning to assess how well various dosages and regimens of the vaccine generate an immune system response in more study subjects and different populations
- Phase 3, the last step before filing for regulatory approval, engages thousands of volunteers with the primary goal of determining whether the vaccine prevents infection or the disease it causes (FDA, 2020)
In addition to establishing a vaccine’s safety and efficacy, developers must provide evidence that adequate process controls will be in place during manufacturing to ensure consistency.
Each part of the R&D process involves its own web of activities that differ around the globe, influenced by diverse sets of actors, regional priorities, health systems, infrastructure capacity, political undercurrents and cultural norms and beliefs. High costs, the challenges of recruiting an adequately representative population, and retaining participants through the duration of a clinical trial are frequent barriers to gathering critical data and completing trials in a timely fashion. Given the many steps involved, a manufacturer will generally not begin to produce a vaccine at volume until success is in sight and regulatory approval is assured. With SARS-CoV-2, however, the government accelerated vaccine development and availability with direct financial support and advance market commitments, incentivizing manufacturers to begin at-scale production prior to completing efficacy trials and long before final regulatory approval without assuming financial risk.
A Network of Players
Vaccine R&D involves researchers, funders, developers, manufacturers, purchasers, payers, regulators, policy makers and program implementers, each influenced to varying degrees by disease burden, technical feasibility, opportunity cost and other factors. There is also the crucial need for ongoing engagement at the community level, beginning at the earliest stages of the R&D process and always with the end user in mind.
The players interact in multiple ways and for a variety of purposes, sometimes contractually and sometimes through other formal or informal collaborations. The research effort, for example, occurs within academic settings and university-affiliated teaching and research hospitals; in government-run laboratories at the National Institutes of Health (NIH) in the United States and similar institutions in Europe, China, India, Russia, Brazil, Cuba, Indonesia, South Korea and elsewhere; in contract research organizations that often have public funding; in major multinational pharmaceutical corporations and other large-scale vaccine producers; and in small biotech companies.

Within the United States, the NIH supports and conducts research across the scientific spectrum, spanning studies in molecular pathogenesis and basic immunology through the clinical testing of candidate vaccines (National Institute of Allergy and Infectious Diseases, 2020). Decades of core support from the NIH and other federal agencies, notably the Department of Defense, yielded the messenger RNA (mRNA) platform technology behind the first two authorized COVID-19 vaccines (Allen, 2020), a compelling testament to the contributions of basic science to vaccine innovation. This commitment to early research and the sorting-out process that is often used to identify and promote encouraging approaches have also lessened the risk of failure for vaccine developers, increasing the odds that they are pursuing more promising candidates.
Established in 2006, the Biomedical Advanced Research and Development Authority (BARDA), under the jurisdiction of the U.S. Department of Health and Human Services, is also a major funder of pandemic vaccine R&D, one focus within its broader mission of safeguarding the nation from potential chemical, biological, radiological and nuclear threats. BARDA was a primary funder of the COVID-19 vaccine jointly developed by NIH and Moderna, which received emergency use authorization from the FDA in December 2020, as well as other COVID-19 vaccines and some of the diagnostic and therapeutic tools in the COVID-19 arsenal (BARDA, 2021).
 Another key player in vaccine R&D is the Coalition for Epidemic Preparedness Innovations (CEPI), launched in 2017 with a mission “to accelerate the development of vaccines against emerging infectious diseases and enable equitable access to those vaccines for people during outbreaks” (CEPI, n.d.). A global public-private partnership, CEPI works on many levels to drive forward the development and deployment of new vaccines. Key activities include stockpiling investigational vaccines with an established safety profile, funding innovative platform technologies to accelerate vaccine development and manufacture when a new pathogen is identified, strengthening response capacity in countries at risk, and advancing regulatory science.
Another key player in vaccine R&D is the Coalition for Epidemic Preparedness Innovations (CEPI), launched in 2017 with a mission “to accelerate the development of vaccines against emerging infectious diseases and enable equitable access to those vaccines for people during outbreaks” (CEPI, n.d.). A global public-private partnership, CEPI works on many levels to drive forward the development and deployment of new vaccines. Key activities include stockpiling investigational vaccines with an established safety profile, funding innovative platform technologies to accelerate vaccine development and manufacture when a new pathogen is identified, strengthening response capacity in countries at risk, and advancing regulatory science.
In the United States, vaccines are reviewed and licensed by the Center for Biologics Evaluation and Research within the FDA. Other key regulatory authorities around the globe are the European Medicines Agency, Japan’s Pharmaceuticals and Medical Devices Agency, Health Canada and Australia’s Therapeutic Goods Administration, each with its own approach to making products available in public health emergencies. Lower- and middle-income countries may not have the capacity to provide rapid expert reviews of novel vaccines and typically rely on evaluations by agencies in other countries. The inefficiencies that come from a lack of harmonized standards for ensuring product safety, efficacy and quality, and the dictates of labeling and packaging, often force developers to meet inconsistent local requirements, and sometimes to conduct additional clinical trials in order to receive regulatory approval (Malvolti & Feiden, 2021).
The WHO, which works at many levels to increase access to vaccines around the world, has tried to ease this problem by coordinating vaccine approvals internationally through its Prequalification Program (WHO, n.d.-c). Established in 2001, the program created a standardized procedure for assessing the quality, safety and efficacy of candidate vaccines (as well as other pharmaceuticals) as a means of supporting and strengthening the capacity of regulatory systems worldwide (Coyne, 2019). The WHO also employs the Emergency Use Listing procedure to assess the suitability of vaccines, therapeutics and diagnostics during public health emergencies. By sharing that information with national regulatory authorities, the WHO enables products to be swiftly authorized for use in countries that have limited resources to conduct their own evaluations (WHO, n.d.-b).
The WHO’s activities fit within a broader equity agenda. Realizing the goals of that agenda requires establishing equity as a core principle and committing to community ownership at every stage of trial design by engaging local researchers from the outset and developing global clinical trial networks that include populations diverse by ethnicity, gender and age. In that context, regional efforts are essential to ensure that lower- and middle-income countries are full partners in the development of vaccines that their populations will ultimately use. The Africa Centres for Disease Control and Prevention Consortium for COVID-19 Vaccine Clinical Trials (CONCVACT), for example, was launched in July 2020 by the African Union Commission to support testing, approval and access to COVID-19 vaccines in Africa (Africa CDC, 2020).
A number of other players have pivotal roles in vaccine procurement and dissemination. While these are separate components of the vaccine/vaccination ecosystem, their work merits mention because they can offer incentives that influence the R&D process — such as advance market commitments for vaccines that meet certain performance criteria.
Gavi, the Vaccine Alliance, provides support for vaccines against 17 infectious diseases, with the goal of increasing access to new and underutilized vaccines in lower- and middle-income countries. Established in 2000 with the WHO, UNICEF, the World Bank and the Bill & Melinda Gates Foundation as core partners, Gavi links donors, national governments, civil society organizations, the pharmaceutical industry, research and technical institutes and others to make high-volume, low-price vaccines widely available (Gavi, the Vaccine Alliance, n.d.). Now the largest vaccine funder in the world, Gavi has unquestionably achieved many of its access goals, but some have questioned whether its pooled procurement and market-shaping activities have put pressure on vaccine prices, posing a potential threat to the industry’s sustainability and capacity to innovate (Watson & Faron de Goer, 2016).
The recognition of the critical need for global coordination in the vaccine response to COVID-19 led to the establishment of COVAX, the vaccine pillar of the Access to Covid Tools Accelerator (ACT-A) (WHO, n.d.-a), which is co-chaired by Gavi, CEPI and the WHO. COVAX is a platform to support the research, development and manufacturing of COVID-19 vaccines; negotiate their pricing; allocate the supply through a formula designed to provide equitable global access regardless of a country’s income level; and support the introduction and rollout of authorized vaccines. The COVAX Facility, the global risk-sharing mechanism for pooled procurement and equitable vaccine distribution, has secured the participation of 191 economies that are either eligible to receive doses or are funding contributors (WHO, 2020d). China joined the COVAX Facility as a procuring participant somewhat after it was initially established, and the United States announced it would contribute funding after President Joe Biden took office, leaving Russia as the only high-income country as of March 2021 that has not opted to participate.
Despite the sophistication and vast reach of the vaccine/vaccination ecosystem, lack of coordination remains one of its enduring characteristics. In a background paper prepared for the group’s meeting, two authors involved in the COVID-19 response laid out the R&D gaps that exist (Lurie & Keusch, 2021). Funders and research groups, they write, were part of a “conductorless orchestra[.] [E]ach … played its part, often exceedingly well, but not always in a way to support an ideal tempo or harmony, or to eliminate needless repetition of movements.” In particular, they point to the lack of predetermined strategies to undertake and share the early enabling scientific data, support the manufacturing of billions of vaccine doses, and guide an equitable, global distribution system. To overcome these gaps, the authors propose the elements of an end-to-end R&D Preparedness and Response Ecosystem.
HIGHLIGHTS OF VACCINE SCIENCE
The R&D that is speeding COVID-19 vaccines through the vaccine/vaccination ecosystem has been years in the making. The technologies in play were launched from a solid base of prior innovation and research investments in a broad array of fields, including structural biology, immunology, virology, oncology, genomics and informatics. Experts across a wide range of disciplines, many without prior training in vaccinology, helped push vaccine development forward, an encouraging sign in a field that has historically been siloed from other areas of research. New vaccine technologies have also benefited from ongoing pandemic preparedness research programs. An example of this innovation is BARDA’s support since 2007 of platforms that use cell cultures and recombinant approaches rather than the more traditional chicken eggs to make seasonal influenza vaccines (Elvidge, 2016). The quest to develop next-generation vaccine production platforms is now being deployed to make COVID-19 vaccines.
Vaccines are highly complex products in no small measure because they generally derive from biological source material, including microorganisms and cellular derivatives, which must be carefully characterized, tested and standardized.
These biological materials must be processed and formulated in specialized manufacturing facilities that have met rigorous quality-control standards. In the United States, the FDA requires product characterization data that include a lot-release protocol identifying the tests that manufacturers must conduct on each batch of vaccines to ensure reliably consistent quality on a lot-by-lot basis (FDA, 2020).
About two-thirds of the vaccines in the COVID-19 pipeline use established technologies, including protein subunit vaccines, replicating and nonreplicating viral vectors and inactivated viruses (Chagar et al., 2021). The remainder use newer technologies that could result in “plug and play” platforms employable against multiple pathogens in the future, such as mRNA and adenovirus vectors, which were pioneered in part from therapeutic drug research programs.
Since its discovery in 1961, mRNA has offered tantalizing promise as a new class of therapy, especially for cancer (Sahin et al., 2014). When used in vaccines directed against SARS-CoV-2, mRNA works by instructing the cells to make a harmless piece of the spike protein found on the surface of the virus. The body generates an immune response to that protein, preventing the virus from infecting human cells. Adenovirus vectors likewise stimulate an immune response, in this case by using a nonreplicating viral vector to deliver the genetic code for the spike protein into cells so that the body can learn to attack it. The adenovirus vector technology has previously been licensed in an Ebola vaccine (European Medicines Agency, 2020).
Both the mRNA and adenovirus approaches to vaccine development had been studied on other emerging viruses, including Zika and MERS, as part of preparedness projects. Funding from the U.S. Department of Defense, BARDA, the NIH, the United Kingdom and CEPI were all key to developing the technologies used in the new vaccines (Hatchett, 2020). The early success of these platforms, as indicated by their safety and efficacy, suggests that future vaccine development efforts may increasingly rely on them because their flexibility allows a working vaccine to be produced based on an organism’s genetic code, offering unprecedented speed and precision.
Whatever the technology, continued scientific progress depends on attracting more investments in vaccines to combat both pandemic and endemic pathogens. Large-scale Phase 3 trials, while still the norm, can be an impediment to that goal because they are costly and time consuming, and no predictable system exists for financing them. Investments in HIV, oncology and immunology have all suggested alternative testing approaches and offer learnings of interest about the potential use of biomarkers and surrogate endpoints that can generate information about efficacy more efficiently without sacrificing standards.
Establishing immunologic correlates of protection could reduce the size and complexity of conducting clinical trials and dramatically lower vaccine development costs and speed regulatory approvals, potentially enhancing commercial appeal for both first-wave vaccine developers and subsequent market entrants. The hope is that making the search for these correlates a priority, and sharing the large datasets and analyses of clinical and laboratory information from COVID-19-infected patients and vaccinated individuals across vaccine development programs, will help to identify novel biomarkers that predict protection. COVID-19 has also highlighted the urgent need to accelerate research efforts on the breadth of host responses to vaccines, including the structure and function of antibodies and other protective components of the immune system.
Even where the nature of the immune correlates are unclear, regulators may be able to accept clinical trial evidence of immune responses that bridge to those of other vaccines. For example, once a given mRNA vaccine demonstrates its ability to prevent COVID-19, regulators could theoretically approve subsequent vaccines that use the same platform if they demonstrate the same immune responses. Such an accelerated pathway will become increasingly important as new viral mutations emerge. That kind of scientific innovation could also incentivize broader participation in vaccine R&D.
VACCINE DEVELOPMENT CHALLENGES AND INCENTIVES
Traditionally, the global pharmaceutical industry has favored products designed to treat disease, rather than to prevent it, since the former typically require multiple doses, are used for longer periods of time and are more likely to produce high returns on their investments.
Until recently, vaccines have not produced similar largesse, in part because they are primarily used by a limited pediatric population and provide needed protection with few doses. That often puts public health at odds with the profits and sustainable business model required by many of the players in vaccine R&D. The remarkable market potential engendered by the COVID-19 pandemic — with billions of people needing vaccines now and perhaps boosters later to maintain immunity and protect against emerging variants — could change this equation, at least for some diseases.
To a for-profit enterprise, the relative disadvantages of investing in vaccines rather than in blockbuster drugs are many, accounting for the dramatic consolidation of the vaccine market since the 1970s (Xue & Ouellete, 2020). Along with their more limited use, vaccines are held to the highest safety standards since they are administered to healthy people, especially healthy infants and children. Effective drugs for serious diseases with limited therapeutic options, by contrast, can sometimes be successfully marketed even when they carry significant risks of a serious side effect.

Moreover, the complexity of the manufacturing process generally makes it necessary to build a new plant for each vaccine. That process can take five years and cost at least $350 million in the United States and $150 million in India (M. Watson, personal communication, November 8, 2020), risks that discourage companies from building a plant until efficacy has been documented in completed clinical trials. Here, too, the new generation of viral vector and mRNA vaccines may change the equation, possibly making it viable to use a single plant to manufacture multiple vaccines.
The barriers to R&D are suggested by an analysis initially performed for the Wellcome Trust that identified 54 challenges, including 16 priority challenges (see Table 2 in Malvolti & Feiden paper, this volume), preventing worthy vaccine candidates from progressing from Phase 2 clinical testing through licensure. Among these challenges are the lack of recognized surrogates or correlates of efficacy, limited regulatory support for adaptive clinical trial design, the lack of harmonized requirements across regulatory agencies, the long lead time needed to establish significant manufacturing capacity, and the lack of partners to receive technology transfer. Pressure from governments and nongovernmental organizations (NGOs) to control price also takes its toll, with the opportunity cost of vaccine development sometimes too high to attract investors who have more potentially profitable options in other diseases and therapeutics, such as diabetes, the chronic diseases of aging and immuno-oncology drugs. Competing pressures on scientific capacity within a company are a further limitation.
While there has been some improvement in predictive capacity along the R&D continuum from animal studies to licensure, discrepancies between immunologic results and determination of efficacy in costly and complex Phase 3 clinical trials can also be stumbling blocks, suggesting the risk of undue reliance on early studies (FDA, 2017) (Figure 1).
Figure 1. Probability of success across phases of vaccine development
Source:
HHS; Biotechnology Innovation Organization; BioMedTracker; Amplion. Clinical Development Success Rates 2006-2015.
Despite these barriers, the vaccine market for the four largest (by value) multinational vaccine producers — GlaxoSmithKline, Merck, Pfizer and Sanofi — has grown sixfold over the past two decades. By comparison, the market for anti-infectives exclusive of vaccines has slightly more than doubled, while the market for oncology drugs has increased thirteenfold (EvaluatePharma, 2021).
Investment Priorities and Procurement Strategies
A background paper drawn from Wellcome Trust research and prepared for the Sabin-Aspen Vaccine Science & Policy Group reviews the many influences on vaccine investment targets, sorting them into the broad categories of technical feasibility, unmet medical need, value creation and strategic fit (Malvolti & Feiden, 2021). While the significance of each factor varies depending on the phase of development and the type of developer, the misalignment between financial incentives and public health needs is often striking.
That is particularly reflected in the disproportionate concentration of vaccine R&D in high-income countries, where four-fifths of the dollar value of the global vaccine market is generated, despite accounting for only one-fifth of the annual volume of vaccines consumed (Shulman et al., 2021). Multinational corporations have generally pursued high-margin vaccines, which are marketed at a relatively low volume and designed to prevent diseases with relatively less global impact on morbidity and mortality, such as an updated pediatric pneumonia vaccine (Malvolti & Feiden, 2021).
 The result is that investment priorities have historically skewed away from endemic diseases that pose their most intense threats to lower- and middle-income populations. For example, a dearth of capital investment has discouraged vaccine R&D to combat malaria and TB. The Ebola virus was well recognized before its aggressive spread, but earlier isolated outbreaks in impoverished regions had never received attention from vaccine developers because the market was limited and the return on investment was expected to be low. The Zika virus, too, had been discovered decades earlier, but the complication of microcephaly in infants born to infected mothers was unknown, and the generally mild forms of the disease from Africa and Asia had not pushed it up the priority list (Billington et al., 2020).
The result is that investment priorities have historically skewed away from endemic diseases that pose their most intense threats to lower- and middle-income populations. For example, a dearth of capital investment has discouraged vaccine R&D to combat malaria and TB. The Ebola virus was well recognized before its aggressive spread, but earlier isolated outbreaks in impoverished regions had never received attention from vaccine developers because the market was limited and the return on investment was expected to be low. The Zika virus, too, had been discovered decades earlier, but the complication of microcephaly in infants born to infected mothers was unknown, and the generally mild forms of the disease from Africa and Asia had not pushed it up the priority list (Billington et al., 2020).
In recent years, the bias against investing in vaccines with large markets but low profit margins has begun to shift as governments, the Bill & Melinda Gates Foundation and Gavi develop strategies to incentivize vaccine R&D and the demand for vaccines. Their combined efforts have helped to increase the number of companies producing basic vaccines for low-income countries from five in 2000 to 18 in 2020 (J. Weintraub, personal communication, November 9, 2020).
The companies within the Developing Countries Vaccine Manufacturing Network (DCVMN) provide another source for R&D and manufacturing capacity. The alliance has brought together some 41 manufacturers from 14 countries and territories, primarily in Asia, Latin America and Africa, which produce vaccines for large, local markets at low prices. DCVMN manufacturers now supply more than 40 different types of vaccines (DCVMN, n.d.). Unlike multinationals, participating companies rely heavily on grants, loans and other funding from public and philanthropic sources. The importance of this effort is reflected in data showing that DCVMN supplies at least half the vaccine doses procured by Gavi and UNICEF in recent years from a variety of manufacturers with a wide range of capacity (Hayman & Pagliusi, 2020).
The pooled procurement mechanisms these and other multilateral agencies employ have proven to be important tools for increasing access to vaccines by generating the volume necessary to attract manufacturers. While some researchers have suggested that the downward pricing pressure they exert on vaccines could ultimately drive manufacturers out of lower- and middle-income markets (Shulman et al., 2021), affordability remains essential to meeting global needs.
Power of Partnerships
The partnerships that have emerged to foster vaccine R&D capacity and access in lower- and middle-income countries — and which especially characterize the global COVID-19 response — have their origins in earlier efforts to combine complementary expertise and capabilities. Examining these partnerships, both those that worked well and those that did not, offers lessons for future initiatives.
The conjugate meningitis A vaccine showcases a successful partnership. The FDA, a Dutch biotech firm, an Indian vaccine manufacturer and the WHO were all involved in its development, with support from the Bill & Melinda Gates Foundation and PATH, an NGO focused on accelerating health equity. Gavi’s role in downstream financing and distribution was also an essential “pull” mechanism that supported vaccine development and production. Intended specifically to prevent recurrent epidemics of meningococcal meningitis in the “meningitis belt” of Africa, a market that was unattractive to other manufacturers, the vaccine was evaluated in countries that later introduced the vaccine. With over 300 million people now vaccinated, the disease has been virtually eliminated in many African nations, preventing tens of thousands of deaths (Sambo et al., 2015).
But partnership-supported endeavors are complex and can also encounter stumbling blocks. The Global HIV Vaccine Enterprise is one example of the need for a reset. The development of an efficacious HIV vaccine faces daunting biological complexities that have not yet been overcome despite more than 30 years of research. Against this background, the enterprise struggled to identify opportunities to significantly influence the overall coordination and strategic direction of the HIV vaccine R&D field and failed to gain meaningful traction. When it became clear that it could not continue as a viable independent organization, it was subsumed into a larger entity, the International AIDS Society, where it primarily focuses on communications and convening efforts for the HIV vaccine field (International AIDS Society, n.d.).

The efforts to accelerate the development of vaccines in response to the 2014-16 Ebola epidemic in West Africa also provide important insights about the value and complexity of public-private partnerships. The absence of shared protocols and the failure of global authorities to communicate how many vaccine doses would be needed, and when, were genuine concerns that needed to be navigated in real time (M. Feinberg, personal communication, July 30, 2020; Wolf et al., 2020). Moreover, the vaccine candidate that ultimately proved to be highly effective in preventing Ebola virus infection (VSV-ZEBOV, now licensed as Ervebo®) had actually been constructed a decade earlier and presumably could have been advanced and ready to deploy much sooner, rather than a year after the epidemic was under way. Unfortunately, the development and investment pathways were not in place at the time of the outbreak, nor were the incentives to attract the necessary partners.
The Ebola epidemic nonetheless offers a number of lessons for vaccine development, including the critical importance of proactive approaches to emerging infectious disease threats and the need for more effective public-private partnership models. The sense of urgency created by the epidemic prompted the FDA to demonstrate more than its routine level of hands-on engagement and regulatory flexibility, just as it did to move COVID-19 vaccines into the market swiftly (Joseph, 2020). By designating the Ebola vaccine as a breakthrough therapy with priority review status, fast-track mechanisms kicked in that allowed the FDA to provide intensive guidance in the earliest phases of clinical trials, ensuring a speedier evaluation. As part of its review process, the agency coordinated with international regulatory agencies and based its approval in part on research conducted outside the United States (Fritz, 2020). The collective recognition of the importance of acting proactively and aligning multisector stakeholders to address global health security threats was a major driver for efforts to establish CEPI.
THE COVID-19 EXPERIENCE
An in-depth look at the record-breaking response to the COVID-19 pandemic, which resulted in several authorized vaccines within a year, is provided elsewhere in this report (Chagar et al., 2021). Perhaps the most important takeaway is that the R&D enterprise is capable of responding at historic speed in the face of an emergency — but even that speed was insufficient to extinguish the threat. The lessons learned from COVID-19 can now be applied not only in pandemics but also, with suitable adaptations, to vaccines that target endemic diseases, especially where existing vaccines would benefit from improved performance. Figure 2 illustrates the development timelines for previously approved vaccines; the hope is to institutionalize strategies to shorten these considerably.
Figure 2. Comparing Vaccine development throughout history
Source: The College of Physicians of Philadelphia; WHO; CDC; National Institutes of Health; Business Insider.
A measure of luck may be part of the COVID-19 success story; the modified spike protein that has been the basis of most major vaccine research thus far appears to be an ideal antigen for priming the immune system. Had the virus featured the immune-system-dodging properties of HIV or some of the other challenges that characterize malaria and TB vaccine development, the outcome could have been very different.
Instead, experience with other coronaviruses (notably, SARS-1 and MERS) enabled researchers to quickly grasp that the spike protein was a promising vaccine target, and cutting-edge technology was ready once the virus was sequenced. Ironically, timing also worked in favor of the COVID-19 vaccine trials. There was so much virus circulating in the United States, the United Kingdom, Brazil and elsewhere that it was possible to compare infection rates in vaccinated and placebo study arms relatively quickly.
However, the appearance of variants capable of evading immunity represents a new challenge that may require other approaches, especially since different vaccines may have varying degrees of efficacy as the virus mutates. Where feasible, trials that allow direct, head-to-head comparisons between vaccines could be of benefit. There has also been a call for broadly protective, “variant-proof” COVID-19 vaccines (Burton & Topol, 2021), which echoes the group’s earlier recommendations for a universal influenza vaccine, which reflects the undiminished threat of an influenza pandemic (Sabin-Aspen Vaccine Science & Policy Group, 2019).

Whatever debt is owed to chance, the vaccines could never have been ready for quick study had it not been for prior investments in basic science and global R&D preparedness. Many other factors also helped to facilitate vaccine development — the urgent threat of the pandemic; an unprecedented willingness to share discoveries and partner on research (Isaacson, 2021); government commitments in the form of financial support and direction, including advance market commitments that greatly lessened financial risk; philanthropic funding; national pride; corporate pursuit of reputational benefit; and regulatory flexibility. Partnerships that had previously been used primarily to advance vaccine R&D in lower- and middle-income countries became ubiquitous, bringing together many combinations of companies, government agencies, academic researchers and NGOs. This was encouraged by the U.S. Department of Justice and the Federal Trade Commission, which issued a joint statement shortly after the pandemic surfaced with guidelines on how usually competitive firms could collaborate without violating antitrust laws (U.S. Department of Justice, 2020), building on the antitrust authority that had been given to BARDA (Pandemic and All Hazards Preparedness Act, 2006).
As of September 2020, about half of all COVID-19 vaccine candidates, including most of those in clinical trials, were being developed through partnerships. Almost half paired two research entities, while just over 40 percent coupled a research entity with a development, manufacturing and commercialization player (Chagar et al., 2021). Pairings included a multinational corporation/biotech partnership and a public sector/private sector team, while other agreements linked multinational corporations, corporate and academic teams, and NGOs and biotechs.
These formal partnerships have been supplemented by a remarkable degree of transparency and a willingness to share results in many quarters. COVID-19 also helped to drive new players into the market. Only 14 percent of the organizations involved in development had previously commercialized vaccines, but many others were not entering the race de novo, having already developed their “pandemic response muscles” through prior experience with Zika, Ebola, MERS, SARS-1, H1N1 or global influenza initiatives (Chagar et al., 2021).
The availability of government and philanthropic funding to develop, produce and purchase COVID-19 vaccines — an estimated $13 billion, excluding China’s investment (Chagar et al., 2021) — has been fundamental to speed. BARDA grants and advance market commitments alone exceed $10 billion (A Bigger Dose, 2020). Normally, each step in vaccine R&D depends on the success of previous steps, as well as evolving market calculations. But with governments removing much of the financial risk while offering technical assistance and brokering partnerships, industry could simultaneously develop and test COVID-19 vaccines while initiating and ramping up preapproval production through at-risk manufacturing.
The United States has also invoked the Defense Production Act to bolster manufacturing infrastructure and ensure there are no gaps in domestic vaccine production. Particularly notable were efforts to secure key ingredients and the partnership brokered by the federal government between Johnson & Johnson (J&J), whose vaccine was authorized in late February 2021, and Merck, which agreed to retool its plants to manufacture additional doses of the J&J vaccine. Government authorities agreed to provide the funding and support needed to expedite the availability of equipment, machinery and supplies; coordinate logistics; and repurpose Merck’s manufacturing facilities (U.S. Department of Health and Human Services, 2021). Weighed against these benefits, however, is the risk that invoking the Defense Production Act may disrupt the global supply chain, impact vaccine production outside the United States and stymie the flow of vaccines across borders. A similar threat to access surfaces in any country that puts export controls in place.
The various financing mechanisms in use, including research funding, advance market commitments and volume guarantees, are well-described in the background papers published elsewhere in this report (Shulman et al., 2021). While these mechanisms have had a transformative influence on the pace of COVID-19 vaccine development, they also raise vigorous and as yet unresolved questions about whether governments should ask more of the companies they support, particularly by requiring them to share data, assays and other information that is traditionally considered proprietary (R. Bright, personal communication, July 23, 2020).
A clearly articulated and streamlined regulatory process proved critical to the pace at which COVID-19 vaccines were developed, just as it had been for Ebola. In the United States, FDA regulators interacted with developers as clinical trials proceeded. The agency’s guidance on efficacy standards, its support for innovative and accelerated clinical trial designs, and the expedited review process all spurred progress. Globally, the WHO’s Emergency Use Listing procedure allowed dozens of countries to authorize the use of COVID-19 vaccines almost immediately after its rapid, rigorous assessment was completed (WHO, 2021a).
The COVID-19 experience has also highlighted R&D gaps, particularly the lack of global governance and the absence of multinational agreements and funding structures (Lurie & Keusch, 2021; Lurie et al., 2021). In general, many countries pursued their own scientific agendas, with no consensus mechanism for developing research priorities or designing clinical trials with either conventional or novel technologies.
Moreover, no-fault vaccine injury compensation systems have not been established in all countries. A mechanism to compensate individuals who experience a serious vaccine-related injury for routinely recommended vaccines exists in only 25 countries — including the United States, much of Europe and the wealthier parts of Asia, but not in Africa (Mungwira et al., 2020). As these liabilities represent a potentially significant risk to manufacturers, establishing programs and policies to shoulder them provides an important incentive for private sector engagement. Without such protection, manufactures are often reluctant to enter these markets.
 While some individual companies reached bilateral immunity agreements with countries that agreed to purchase their COVID-19 vaccines, no entity was initially responsible for ensuring that liability policies and vaccine injury compensation programs were in place prior to vaccine introduction. Recognizing that the COVAX commitment to distribute vaccines in lower-income countries could have been jeopardized without liability protections (Halabi et al., 2020), COVAX created a new program to compensate eligible individuals in 92 lower- and middle-income countries without a need to resort to courts of law. This program, funded by a small levy on each dose, is the first and only vaccine injury compensation mechanism established on a global scale (WHO, 2021b). Going forward, such a system should not have to be reinvented for the next global public health emergency.
While some individual companies reached bilateral immunity agreements with countries that agreed to purchase their COVID-19 vaccines, no entity was initially responsible for ensuring that liability policies and vaccine injury compensation programs were in place prior to vaccine introduction. Recognizing that the COVAX commitment to distribute vaccines in lower-income countries could have been jeopardized without liability protections (Halabi et al., 2020), COVAX created a new program to compensate eligible individuals in 92 lower- and middle-income countries without a need to resort to courts of law. This program, funded by a small levy on each dose, is the first and only vaccine injury compensation mechanism established on a global scale (WHO, 2021b). Going forward, such a system should not have to be reinvented for the next global public health emergency.
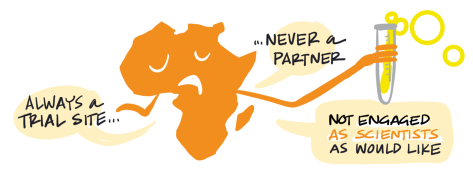
Wealthier countries dominate the vaccine R&D sphere, with 70 percent of the research effort led from high-income countries and the remainder from upper-middle-income countries, particularly China and India. South Africa has also played a major role, but a very limited number of clinical trials are taking place elsewhere in Africa, continuing a troubling tradition of excluding the continent from opportunities to gather data on its own population and undermining efforts to strengthen clinical trial capacity (Makoni, 2020; COVID-NMA, n.d.) (Figure 3).
Figure 3. COVID-19 vaccine clinical trials
Source: Romain Vuillemot – LIRIS, École Centrale de Lyon; Philippe Rivière – LIRIS, VisionsCarto; Pierre Ripoll – LIRIS, INSA Lyon; Julien Barnier – Centre Max Weber, CNRS. Retrieved from: https://covid-nma.com/vaccines/mapping/ [Online Resource]
Much remains uncertain about future progress against COVID-19. Already by the end of 2020, companies with successful vaccines were facing conflicting pressures to unblind and vaccinate participants in the placebo arms or to maintain them to provide longer-term efficacy and safety data (Cohen, 2020). Subsequent vaccine candidates are likely to be compared to vaccines already on the market, but the 95 percent efficacy rates of the first two U.S.-authorized products set an exceptionally high bar globally. Many vaccine developers are also concerned about the availability of resources since BARDA, CEPI and other funders do not typically support late-stage clinical trials (Chagar et al., 2021). And the emergence of viral variants raises new concerns about the breadth and duration of vaccine protections and the potential need for booster shots or other adaptations.
Unfortunately, though safe and effective, the earliest vaccines authorized in the United States were not ideal for meeting the world’s needs because they involve two injections several weeks apart and require complex cold storage, come with relatively high prices and are initially limited by manufacturing capacity. Other vaccine candidates are working their way through the development pipeline, and the U.S.-authorized J&J vaccine, with its simpler storage requirements and single-shot administration, broadens the supply. Nonetheless, there is still concern that first-up vaccines can establish a stranglehold on the market, disadvantaging subsequent vaccine R&D projects that may have other benefits.

These issues are not unique to COVID-19 vaccines, as the broad use of vaccines against pertussis and influenza illustrates. In those examples, their status as the standard of care in a widely vaccinated population makes it challenging for potentially more effective formulations to break through; they are likely to do so only if they can translate into a significant return on investment for vaccine developers.
KEY THEMES
The aggressive and remarkable response to the SARS-CoV-2 virus may well open the door to a new era of vaccines. Expectations could not be higher, given the hope that vaccines will dramatically alter the trajectory of the COVID-19 pandemic and restore a degree of normalcy to life. The convergence of innovations in science and technology with the first-in-a-century worldwide pandemic offers a unique opportunity to establish a new R&D model for pandemics and for the serious infectious diseases that lack vaccines, as well as for innovative vaccines that offer meaningful improvements over those that already exist.
The task ahead is to provide the framework and incentives for standardized approaches to vaccine R&D that encourage continuous streams of innovation while disrupting the cycle of avoidance and neglect that have long characterized the global response to infectious disease. Doing all of that requires strengthened processes, policies and institutions, as well as consistent funding.
In that spirit, these key themes shaped the deliberations of the Sabin-Aspen Vaccine Science & Policy Group:
- Developing and having timely access to safe and effective vaccines for all people is a moral imperative. As a foundational component of primary health care systems and a driver of universal health coverage, vaccines impact every sector of society, reaching more people than any other health or social service (Kelleher, 2020). Indeed, they are essential to achieving the 2030 Agenda for Sustainable Development advanced by the United Nations (United Nations, n.d.) and are a core component of the Immunization Agenda 2030, the global vision to ensure that everyone can benefit fully from vaccines, which has been endorsed through the World Health Assembly (WHO, 2020a).Yet inequities complicate the R&D component of the vaccine/vaccination ecosystem at every turn. In addition to their immediate impact on the global supply of COVID-19 vaccines, the dominance of wealthier countries in the research, regulatory approval and manufacturing processes fosters inequities in the choice of diseases that become the target of vaccine R&D, the ways in which new vaccines are tested, and access to lifesaving products once they become available.
- The devastation wrought by COVID-19 demands that we revisit and restructure the vaccine R&D enterprise. The rapid response of governments, philanthropies, NGOs, companies large and small and the scientific community has shown how the status quo can be modified to move vaccines rapidly through the pipeline. Recognizing the impact of the pandemic on the world’s health, social fabric and economic stability, these same players must catalyze the opportunity for transformative change. The effective COVID-19 vaccines that were produced in record time offer a template for revisiting — and perhaps revamping — current approaches. Relatively small infusions of capital from public and private sources enabled development of novel platforms prior to the pandemic, which could then be deployed against the pathogen when it emerged. The roles of dedicated partnerships and goal-oriented funding also suggest strategies for concentrating R&D attention on endemic infectious diseases, albeit on a modified timeline.
- Scientific breakthroughs and new technology expand ideas of what is possible. Prior investments in basic science allowed researchers to sequence the SARS-CoV-2 virus, synthesize the spike protein, choose it as the target of a vaccine candidate, prepare it for the mRNA and other platforms and launch human trials within six weeks (Lurie & Keusch, 2021). Those remarkable accomplishments demonstrated the potential of mRNA to create “bespoke protein factories” (Johnson, 2020), one among many examples of how the pandemic’s urgency connected seemingly disparate scientific fields. By March 2021, 78 different COVID-19 vaccine trials employing a host of technologies were under way, 22 in Phase 3 (Zimmer et al., 2021).These approaches remind us of the vital need to continue investing in basic science. They also highlight the value of developing multiple vaccines that take different approaches to the same pathogen, given the difficulty of predicting which vaccine is best suited for a particular disease threat. Although these kinds of efforts may not pay off in the short term — or indeed ever — they nonetheless represent important public health and clinical opportunities, prudent use of financial resources and potential opportunities for profit. As well, they tee up the importance of regulatory science that can keep pace with novel trial designs as they accrue data. COVID-19 is a case study in the ability to streamline regulation without sacrificing quality.
- A top-down, bottom-up approach, guided by coordinated leadership at all levels, advances vaccine R&D most effectively. Individuals working across different disciplines, organizations and governments are critically important to the R&D effort, but none operates optimally alone. Vaccine R&D is a team sport, one that requires champions and a mix of innovative, self-motivated groundwork and organized systems within a leadership structure that integrates the knowledge and experience of all contributors.The current model of a “conductorless orchestra” has failed to provide the coherent end-to-end strategy essential to efficient vaccine R&D. While space needs to be available for unstructured, out-of-left-field discoveries, conductors of some sort are also needed to guide the many players within a larger framework so that ideas can bubble up, projects can be linked and gaps can be identified to achieve essential goals.
- Public confidence in the R&D process is imperative to the ultimate
 goal of reducing the toll of vaccine-preventable diseases. Trust in the integrity of every component of the vaccine/vaccination ecosystem — assurance that science and public health concerns are paramount drivers of the research, development, approval and distribution process — safeguards the entire enterprise. Confidence elevates support for research, including basic science, and ensures widespread use of safe and effective vaccines once they are available. The rise in vaccine hesitancy explored in the group’s previous report, Meeting the Challenge of Vaccination Hesitancy (Sabin-Aspen Vaccine Science & Policy Group, 2020), threatens to undermine progress in vaccine development as well as vaccination. The recommendations in that report, including building a strategic narrative that focuses on the achievements and promise of vaccinations, are designed to generate the confidence essential to widespread adoption of safe and effective vaccines.
goal of reducing the toll of vaccine-preventable diseases. Trust in the integrity of every component of the vaccine/vaccination ecosystem — assurance that science and public health concerns are paramount drivers of the research, development, approval and distribution process — safeguards the entire enterprise. Confidence elevates support for research, including basic science, and ensures widespread use of safe and effective vaccines once they are available. The rise in vaccine hesitancy explored in the group’s previous report, Meeting the Challenge of Vaccination Hesitancy (Sabin-Aspen Vaccine Science & Policy Group, 2020), threatens to undermine progress in vaccine development as well as vaccination. The recommendations in that report, including building a strategic narrative that focuses on the achievements and promise of vaccinations, are designed to generate the confidence essential to widespread adoption of safe and effective vaccines.
FIVE BIG IDEAS TO POWER VACCINE R&D
While the core components of the vaccine/vaccination ecosystem have served us well in many ways, the pressing threats of emerging and long-standing diseases, coupled with novel science and technology, call for further action. Based on its deliberations and the themes that emerged from them, the Sabin-Aspen Vaccine Science & Policy Group offers five big ideas to overhaul R&D and establish a new normal to accelerate the availability of vaccines to combat both pandemic and endemic diseases:
Define leadership roles, responsibilities and mechanisms of accountability to prepare for the R&D demands that surface in a pandemic. The global organizations that now have core roles to play in vaccine R&D should map out a real-time vaccine response so that they are ready to act as infectious diseases emerge or spread. A multipronged structure that anticipates R&D needs before they become acute and delegates responsibility for each part would be the matching bookend to the coordinated efforts represented by COVAX, which was developed to ensure access to vaccines around the world once they are approved. An explicit, well-defined strategy — essentially, a flowchart of who is to do what and when, and who is ultimately accountable for each action — is essential so that vaccine R&D is timely, streamlined, equitable and comprehensive.

The speed at which COVID-19 vaccine R&D moved forward amply demonstrates the power of cooperation and vast resources but also highlights limitations. Mechanisms for sharing newly discovered viral strains and subsequent mutations, intellectual property rights and liability protection all proved to be “orphan” problems — no one was responsible or accountable for them. Each potential showstopper had to be addressed under the intense pressure of a pandemic. Likewise, strategies for forging partnerships to develop and swiftly test new vaccines in lower- and middle-income countries and opportunities for the efficiencies of regulatory harmonization had not been the focus of advance planning.
The group does not believe a new entity is required to write the rules of engagement so that these and other challenges can be considered in a measured way. Rather, that work should be undertaken by a multistakeholder alliance of institutions already engaged in the vaccine/vaccination ecosystem that come together to reach consensus on a systems approach.
These partners should convene in structured planning sessions to determine the key issues that need to be addressed and to make decisions about who will be responsible for taking them on. Participants also need to devise clear lines of communication, determine how they will intersect with one another as needs evolve and ensure adequate capacity, including funding and human resources. A truly global consensus on how to move forward, framed around the belief that vaccines are a public good, will require a multicentric leadership model that engages partners not only in the United States and Europe but also in China, Brazil, India and the African nations. Attention to aligning incentives for furthering vaccine R&D should be a priority for this convening (see the last big idea, below).
With the key players collaborating, a similar consensus-building process to advance vaccine R&D for endemic diseases should be pursued. Here, too, the goal of negotiating and assigning roles and responsibilities, and establishing accountability, is to streamline the R&D process. A systems approach has the potential to surface opportunities for efficiencies and processes that can shorten timelines and lower costs, making public health investments more viable and commercial pursuit more tempting.
Propel a transdisciplinary research effort built around partnerships to expand and advance vaccine science. Nothing in recent times has showcased the payoff of basic science investment as much as COVID-19 vaccines — a “decades-long overnight success story” that drew on years of earlier research to confront a devastating new virus. The breakthrough in platform science reflected in the mRNA delivery system was especially noteworthy, but it is not alone. That vaccines are being delivered and beginning to change the trajectory of the pandemic in less than a year is a testament to the power of partnerships to break down institutional and competitive barriers to scientific collaboration.
Such success can only become routine with intentional efforts to engage people across fields and create space for the kind of serendipity that drives novel research and transformational science. A transdisciplinary research effort should combine basic scientific advances in immunology, genomics, microbiology and vaccinology with the latest in computational, engineering and physical sciences, bringing them together in an environment designed to stimulate creative problem-solving and direct science and technology to unanswered questions. The Bill & Melinda Gates Foundation offers a model for what we have in mind: its Global Grand Challenge for Universal Influenza Vaccine Development seeks “completely transformative approaches, rather than incremental research,” and calls for “interdisciplinary collaboration and cross-fertilization from outside the traditional influenza research community” (Global Grand Challenges, 2018).
The Vaccine Group’s report, Accelerating the Development of a Universal Influenza Vaccine (Sabin-Aspen Vaccine Science & Policy Group, 2019), calls for developing and implementing a directed research agenda to drive innovation. The Sabin Institute’s Influenzer Initiative grew out of that report with a commitment to a convergent scientific agenda that merges “expertise from life sciences with physical, mathematical, and computational sciences, and with engineering—a blueprint for innovation that both builds on fundamental knowledge and stimulates novel, cross-cutting discoveries” (Influenzer Initiative, n.d.). The goal is to integrate existing knowledge and shape new frameworks of inquiry to catalyze fresh discoveries, breakthrough developments and their translation into viable vaccines.
To promote convergence science in the broader vaccine R&D enterprise, the group recommends measures to:
- Foster unexpected discoveries across disciplines that have historically been distinct from one another in both knowledge and methods. The Influenzer Initiative has identified the following scientific fields as offering promising opportunities to cross-pollinate and inform vaccine R&D: synthetic biology, bioengineering, systems biology, biophysics, artificial intelligence/machine learning, bioimaging, bioinformatics/computational modeling, chemical engineering and immuno-oncology.
- Bring together funding mechanisms, leadership, and oversight to support a research infrastructure that can advance vaccine science and create opportunities for the novel and risky ideas that percolate from the ground up.
- Leverage lessons from new scientific areas to foster innovation, including improved understanding of the chemistry and physics of vaccine formulation, the immunologic basis of protection and correlates of protective outcomes.
- Distinguish between the intensity of the R&D efforts needed in the face of a potential pandemic and those for long-standing endemic diseases. Research in one arena should inform the other, allowing resources to be allocated as efficiently as possible.
- Ensure that an incentive structure exists to promote and support transdisciplinary research (as discussed in the last big idea, below).
Reimagine clinical trials. Clinical trial design is ripe for more efficient and nimbler approaches. The high evidentiary barriers currently required to establish vaccine safety and efficacy have limited innovation and commercial motivation to pursue new products. In particular, the large-scale enrollment and long duration required for Phase 3 trials have been impediments. It is possible to develop better systems without compromising standards — the pace at which researchers assembled a compelling body of evidence for Ebola and COVID-19 vaccines suggests ways to overhaul current practices.
Equity and efficiency must be prime drivers. While vaccines for most endemic diseases enroll large numbers of participants from lower- and middle-income countries — because that is where the diseases most often occur and where vaccines can be evaluated in field settings — enrollment has been much less robust where high-income countries also have significant disease burdens, as is the case with COVID-19. Countries with more limited resources must not only have their populations well represented among study subjects but also must become full partners in clinical trial development from its inception. Developing research capacity and local trial networks, ensuring that the questions under investigation are relevant to local needs, and sharing results with the affected communities are all essential to establishing a sense of shared ownership in the studies.
Because it may be difficult to predict which approach is best suited for a particular disease threat, another important priority is encouraging vaccine trials that take aim at the same pathogen in different ways. The present system often forces promising candidates to drop out after a competitor succeeds, because the barriers to testing subsequent generations of vaccine become too high. Alternative strategies are needed to encourage a more dynamic market, especially after first-generation products become available. These strategies include:
- Establishing global networks of clinical trial sites for vaccines. In the interests of equity, good science and timely results, these sites must fully reflect the diversity of the world’s people and be prepared to enroll participants as needs arise. Integrating knowledge as it accumulates around the world and pooling control groups will speed the data-gathering process and increase confidence and trust in trial findings.
- Making the identification of immune correlates of protection a priority. Bringing together all of the large datasets and analyses of clinical and laboratory information on infected and vaccinated individuals is the pathway to this critical goal. Once such correlates are identified, vaccines can be approved on the basis of smaller and faster trials that bridge from surrogate endpoints established from initial efficacy studies. The absence of clear laboratory measures that equate to a degree of immunity poses a recurring barrier to this goal.
- Advancing the use of master protocols in order to accelerate vaccine trials and make the findings more comparable, with careful attention to strategies that do not discourage innovation.
- Considering opportunities for adaptive trial designs that incorporate a “review and adapt” component at the time studies are launched. By planning for interim scrutiny of accumulating data and employing innovative statistical techniques, researchers may be able to measure treatment effects more quickly, abandon futile treatment arms, establish optimal dosage and refine their knowledge about who is most likely to benefit from a treatment (Pallmann et al., 2018).
- Assessing the value and appropriate use of human challenge trials. In such trials, participants are deliberately exposed to a pathogen in a controlled setting to speed knowledge about vaccine efficacy. Considering how best to design such trials, and when and where they can be used to advance science, is essential to developing suitable guidelines for implementing them.
- Structuring clinical trials to inform future programmatic and policy decisions as well as regulatory approval. Trials that evaluate efficacy in subpopulations categorized by age, gender, illness status, race and ethnicity yield important data that can be used to ensure that vaccines are developed with the end user in mind. Trials also need to answer questions relevant to population impact, such as who will benefit most from immunization and the interchangeability of different vaccines against the same disease. And trial designs that use the same methodology and outcome measures will make head-to-head comparisons of efficacy more viable.
- Expanding well-funded postmarketing studies to assure long-term safety and effectiveness and optimal use at a population level, especially where the trials that inform vaccine approval enroll small populations or are based on short-term findings. The tools used to evaluate safety and effectiveness and to collect data should be standardized for lower-, middle- and high-income settings.
- Pairing postmarketing safety surveillance with a commitment to advancing vaccine safety science. Understanding the mechanisms of serious adverse events following immunization and identifying “correlates of safety” before they surface in large, population-based monitoring can generate actionable findings more rapidly.
Restructure regulatory science to reflect advances in vaccine R&D. Transformative vaccine discoveries and technology must be accompanied by equally transformative approaches to regulatory science and process. Extensive regulatory requirements are in place not only to assess clinical trial data but also to assure that effective, safe and consistent products emerge from the manufacturing process, especially as the volume scales up. Without compromising safety and efficacy, regulatory science innovations should be pursued to streamline preclinical evaluation; make vaccine trials faster, nimbler and more cost-effective; and enhance product scale-up, manufacturing and postmarketing surveillance. Steps include:
- Harmonizing global regulatory standards and approaches, including requirements for surrogate endpoints and postmarketing surveillance, with strategies in place to provide the training, technical assistance and resource mobilization that regulators in lower- and middle-income countries may need. As well, emphasize consistent documentation requirements and further strengthen the reliance mechanisms established by the WHO for rapid authorization of COVID-19 vaccines to relieve unnecessary burdens on developers.
- Distinguishing between vaccine approval standards that apply only to emerging pathogens and potential pandemics and those applicable to all vaccines. Antitrust waivers, for example, may be appropriate only in emergency situations.
- Examining current regulatory review processes under the FDA’s Emergency Use Authorization, the emergency measures permitted by European Medicines Agency, and the WHO Emergency Use Listing procedure, with an eye toward any necessary improvements.
- Developing training to strengthen and extend the global availability of expertise, especially to build regulatory capacity in lower- and middle-income countries and a broader pool of vaccine safety experts and systems to assess adverse events comprehensively.
Position vaccines as a public good and align incentives so that benefits accrue to all sectors of society. Much like a utility, vaccines are a public good primarily in the hands of the private sector. That perspective suggests opportunities to propel R&D forward, building on the recognition that the public everywhere has an interest in the timely availability of safe and effective vaccines and that the companies expect to be rewarded for producing them. Strategies for incorporating this “public good” framework into R&D decision making should be part of the strategic thinking undertaken at the leadership convening proposed in the first big idea, above.

Because we have failed to treat vaccines as a shared interest, incentives to advance vaccine R&D have often been misaligned. Historically, the priorities of public health have not been in sync with those of vaccine developers, and commercial players have resisted attempts by government to establish research priorities that might not align with their own strategic plans or portfolios. For companies motivated primarily by profit, this is particularly evident in the mismatch between the locations where vaccine-preventable diseases are most prevalent and where customers are most able to pay for vaccines. Other evidence of misalignment goes beyond the private sector — for example, the system of academic advancement often serves to discourage researchers from sharing their findings prior to publication.
When the full health and economic value of vaccines to individuals and society is recognized, different considerations come to the fore. Examining the incentives that are currently in place is the springboard for actions to better align them. While the right strategies need further study, the COVID-19 pandemic offers an opening, having clearly demonstrated the value of inducements that encourage key players to pursue an urgent, singular goal. In those historic circumstances, governments and philanthropies helped to reduce risk, promote partnerships and provide the financial and reputational benefits that inspired commitments.
There are other precedents for this as well, notably the incentives that were developed to entice researchers and companies to study therapies for orphan drugs. Marketing exclusivity, grants and tax credits have all played a role in significantly increasing the number of orphan drugs that have come to market since the Orphan Drug Act was passed in 1983 (Jozst, 2019).

The challenge now is to identify and exploit similar opportunities for other vaccine-preventable diseases in both emergent and nonemergent situations. Agreeing on the research agenda is clearly a front-and-center goal. Manufacturers, researchers and other core partners need to be incentivized to pursue vaccine R&D that takes aim at the infectious diseases posing the greatest risk to regional needs and the broader global community. The nature of those incentives will vary, depending on the respective roles and action drivers of each player and on the urgency of the public health challenge, but reward mechanisms need to be designed by those in a position to offer them — government, philanthropy and academic institutions.
There should also be a strong push to develop or maintain policies and practices that promote information sharing. Several leading vaccine makers released the protocols for their Phase 3 COVID-19 trials, a strategy that should establish precedent rather than being viewed as a special case.
To guide alignment, criteria are needed to help determine which incentives should be offered and under what circumstances and when government requirements may be necessary. The relative urgency of the disease threat is obviously a significant influence. Incentives for manufacturers can include regulatory flexibility, expedited review, guaranteed market share and intellectual property and liability protections. For researchers, opportunities for funding and career advancement may be the most enticing incentives, suggesting that institutional leaders can tie promotion, tenure, awards and other professional accolades to investigations focused on areas of greatest need.
Full consideration to aligning incentives should be part of the proposed leadership convening described above in the first big idea. Importantly, any offer of incentives should demand something back. Public and philanthropic investments in vaccine partnerships and research initiatives should be conditioned on broadening access to the returns of those investments, such as by sharing data, protocols and findings.
MOVING FORWARD
The big ideas proposed here should be a critical part of any effort to reexamine and restructure the R&D component of the vaccine/vaccination ecosystem. COVID-19 has brought the need for innovation and equity — not only in technology but also in surveillance strategies, partnerships, access to data, genomic sequencing, trial protocols and so much more — sharply to the fore. Experiences with vaccine R&D for numerous other infectious diseases also have much to teach us about what can go right, how to avoid pitfalls and what needs to happen to confront both future pandemics and perennial pathogen threats. Time and preparedness, it is clear, are of the essence to prevent the shattering impact of global pandemics — as well as the chronic devastation of ongoing infectious disease around the globe.
While COVID-19 will offer lessons for some time to come, we have already learned enough to begin to establish new R&D norms. The Sabin-Aspen Vaccine Science & Policy Group looks forward to working with all of those involved with restructuring the vaccine/vaccination ecosystem to move this agenda forward in creating a new normal for vaccine R&D.
References
Africa Centres for Disease Control and Prevention. (2020, July 9). Africa Union Commission launches consortium for COVID-19 vaccine clinical trial [Press release]. https://africacdc.org/news-item/african-union-commission-launches-consortium-for-covid-19-vaccine-clinical-trial/
Allen, A. (2020, November 18). For billion-dollar COVID vaccines, basic government-funded science laid the groundwork. Scientific American. https://www.scientificamerican.com/article/for-billion-dollar-covid-vaccines-basic-government-funded-science-laid-the-groundwork/
A bigger dose: The world is spending nowhere near enough on a coronavirus vaccine. (2020, August 8). The Economist. https://www.economist.com/leaders/2020/08/08/the-world-is-spending-nowhere-near-enough-on-a-coronavirus-vaccine
Billington, J., Deschamps, I., Erck, S. C., Gerberding, J. L., Hanon, E., Ivol, S., Shiver, J. W., Spencer, J. A., & Van Hoof, J. (2020). Developing vaccines for SARS-Cov-2 and future epidemics and pandemics: Applying lessons from past outbreaks. Health Security, 18(3), 241–249. https://doi.org/10.1089/hs.2020.0043
Biomedical Advanced Research and Development Authority. (2021, March 11). Public Health Emergency. https://www.phe.gov/about/barda/Pages/default.aspx
Bright, R. (2020, July 23). Interview with Vaccine Science & Policy Group staff.
Burton, D. R., & Topol, E. J. (2021, February 8). Variant-proof vaccines — invest now for the next pandemic. Nature. https://www.nature.com/articles/d41586-021-00340-4
Bush, V. (1945). Science, the endless frontier. Office of Scientific Research and Development. https://www.nsf.gov/od/lpa/nsf50/vbush1945.htm
Centers for Disease Control and Prevention. (1999). Ten great public health achievements — United States, 1900–1999. Morbidity and Mortality Weekly Report, 48(12), 241–264. https://www.cdc.gov/mmwr/pdf/wk/mm4812.pdf
CEPI. (n.d.). Why we exist. Retrieved March 25, 2021, from https://cepi.net/about/whyweexist
Chagar, A., Thomas, M., Zuo, L., & Watson, M. (2021). The R&D response to COVID-19: What can we learn for the vaccine ecosystem? Powering Vaccine R&D: Opportunities for Transformation. Sabin-Aspen Vaccine Science & Policy Group.
Cohen, J. (2020, December 22). Makes of successful COVID-19 vaccines wrestle with options for placebo recipients. Science. https://www.sciencemag.org/news/2020/12/makers-successful-covid-19-vaccine-wrestle-options-many-thousands-who-received-placebos
COVID-NMA. (n.d.). COVID-19 – living NMA initiative. Retrieved March 23, 2021, from https://covid-nma.com/vaccines/mapping/
Coyne, P. E. (2019). The World Health Organization Prequalification Programme—playing an essential role in assuring quality medical products. International Health, 112(2), 79–80. https://doi.org/10.1093/inthealth/ihy095
Danzon, P., & Pereira, N. S. (2005). Why sole-supplier vaccine markets may be here to stay. Health Affairs, 24(3), 694–756. https://doi.org/10.1377/hlthaff.24.3.694
Developing Countries Vaccine Manufacturing Network. (n.d.). About. Retrieved March 23, 2021, from https://dcvmn.org/-About-
Duffy, J. (1992). The sanitarians: A history of American public health. University of Illinois Press.
Dumonteil, E., Herrera, C., & Buekens, P. (2019). A therapeutic preconceptional vaccine against Chagas disease: A novel indication that could reduce congenital transmission and accelerate vaccine development. PLoS Neglected Tropical Diseases, 13(1), Article e0006985. https://doi.org/10.1371/journal.pntd.0006985
Elvidge, S. (2016, September 23). Protein sciences lands BARDA grant for pandemic flu. BioPharma Dive. https://www.biopharmadive.com/news/protein-sciences-lands-barda-grant-for-pandemic-flu/426180/
European Commission. (2020, December 11). Questions and answers: Conditional marketing authorisation of COVID-19 vaccines in the EU. https://ec.europa.eu/commission/presscorner/detail/en/qanda_20_2390
European Medicines Agency. (2020, July 23). Zabdeno. https://www.ema.europa.eu/en/medicines/human/EPAR/zabdeno
EvaluatePharma. (2021, March). Evaluate. https://www.evaluate.com/ Retrieved from evaluate.com; McKinsey analysis.
Feinberg, M. (2020, July 30). Interview with Vaccine Science & Policy Group staff.
Fritz, M. (2020, February 11). Drug approval during a public health crisis. Regulatory Review. https://www.theregreview.org/2020/02/11/fritz-drug-approval-during-public-health-crisis/
Gavi, the Vaccine Alliance. (n.d.). Our alliance. Retrieved March 23, 2021, from https://www.gavi.org/our-alliance
Global Grand Challenges. (2018). Ending the pandemic threat: A grand challenge for universal influenza vaccine development. https://gcgh.grandchallenges.org/challenge/ending-pandemic-threat-grand-challenge-universal-influenza-vaccine-development
Halabi, S., Heinrich, A., & Omer, S. B. (2020, December 3). No-fault compensation for vaccine injury—the other side of equitable access to Covid-19 Vaccines. New England Journal of Medicine, 383:e1250(0), null. https://doi.org/10.1056/NEJMp2030600
Hatchett, R. (2020, October 16). Testimony before National Vaccine Advisory Committee, US Department of Health and Human Services. https://www.hhs.gov/vaccines/nvac/meetings/2020/10-16/index.html
Hayman, B., & Pagliusi, S. (2020). Emerging vaccine manufacturers are innovating for the next decade. Vaccine: X, 5. https://doi.org/10.1016/j.jvacx.2020.100066
Influenzer Initiative. (n.d.). Building a convergence science agenda for next-generation, universal influenza vaccines. Retrieved March 24, 2021, from https://www.influenzer.org/resources/convergence-science-agenda/csa-brief
International AIDS Society. (n.d.). Global HIV Vaccine Enterprise Strategic Plan, 2018–2023. https://www.iasociety.org/web/webcontent/file/GHVE_Strategic_Plan_2018.pdf
Isaacson W. (2021, March 3). CRISPR rivals put patents aside to help in fight against COVID-19. STAT. https://www.statnews.com/2021/03/03/crispr-rivals-put-patents-aside-fight-against-covid-19/
Johnson, C. Y. (2020, December 6). A gamble pays off in ‘spectacular success’: How the leading coronavirus vaccines made it to the finish line. Washington Post. https://www.washingtonpost.com/health/2020/12/06/covid-vaccine-messenger-rna/
Joseph, A. (2020, July 30). “A huge experiment”: How the world made so much progress on a COVID-19 vaccine so fast. STAT. https://www.statnews.com/2020/07/30/a-huge-experiment-how-the-world-made-so-much-progress-on-a-covid-19-vaccine-so-fast/
Joszt, L. (2019, January 4). 5 things about the Orphan Drug Act. American Journal of Managed Care. https://www.ajmc.com/view/5-things-about-the-orphan-drug-act
Kelleher, K. (2020). Achieving the Sustainable Development Goals: Six reasons why immunization matters. Gavi, the Vaccine Alliance. https://www.gavi.org/achieving-the-sustainable-development-goals-six-reasons-why-immunisation-matters
Lurie, N., & Keusch, G. T. (2021). Designing an R&D preparedness and response ecosystem for potentially pandemic pathogens. Powering Vaccine R&D: Opportunities for Transformation. Sabin-Aspen Vaccine Science & Policy Group.
Lurie, N., Keusch, G. T., & Dzau, V. J. (2021). Urgent lessons from COVID-19: Why the world needs a standing, coordinated system and sustainable financing for global research and development. The Lancet, 397(10280), 1229–1236. https://doi.org/10.1016/S0140-6736(21)00503-1
Makoni, M. (2020). COVID-19 vaccine trials in Africa. The Lancet Respiratory Medicine, 8(11), E79–E80. https://doi.org/10.1016/S2213-2600(20)30401-X
Malvolti, S., & Feiden, K. (2021). Understanding the vaccine ecosystem: Structure and challenges. Powering Vaccine R&D: Opportunities for Transformation. Sabin-Aspen Vaccine Science & Policy Group.
Mungwira, R. G., Guillard, C., Saldaña, A., Okabe, N., Petousis-Harris, H., Agbenu, E., Rodewald, L., & Zuber, P. L. F. (2020). Global landscape analysis of no-fault compensation programmes for vaccine injuries: A review and survey of implementing countries. PloS One, 15(5), Article e0233334. https://doi.org/10.1371/journal.pone.0233334
National Institute of Allergy and Infectious Diseases. (2020, August 13). Vaccines. National Institutes of Health, U.S. Department of Health and Human Services. https://www.niaid.nih.gov/research/vaccines
Pallmann, P., Bedding, A. W., Choodari-Oskooei, B., Dimairo, M., Flight, L., Hampson, L., Holmes, J., Mander, A. P., Odondi, L., Sydes, M. R. Villar, S. S., Wason, J. M. S., Weir, C. J., Wheeler, G. M., Yap, C., & Jaki, T. (2018). Adaptive designs in clinical trials: Why use them, and how to run and report them. BMC Medicine, 16(29). https://doi.org/10.1186/s12916-018-1017-7
Pandemic and All Hazards Preparedness Act, Pub. L. No. 109–417, 120 Stat. 2831. (2006). https://www.govinfo.gov/content/pkg/PLAW-109publ417/pdf/PLAW-109publ417.pdf
Biomedical Advanced Research and Development Authority (BARDA). (2021, March 11). Public Health Emergency. Retrieved from https://www.phe.gov/about/barda/Pages/default.aspx
Sabin-Aspen Vaccine Science & Policy Group. (2019). Accelerating the development of a universal influenza vaccine. https://www.influenzer.org/app/uploads/2020/01/sabin-aspen-report-digital.pdf
Sabin-Aspen Vaccine Science & Policy Group. (2020). Meeting the challenge of vaccination hesitancy. https://www.sabin.org/sites/sabin.org/files/sabin-aspen-report-2020_meeting_the_challenge_of_vaccine_hesitancy.pdf
Sahin, U., Kariko, K., & Tureci, O. (2014). mRNA-based therapeutics—developing a new class of drugs. Nature Reviews Drug Discovery, 13, 759–780 https://doi.org/10.1038/nrd4278
Sambo, L., Chan, M., Davis, S., Lake, A., Berkley, S., Poonawalla, C., & Elias, C. J. (2015). A vaccine meets its promise: Success in controlling epidemic meningitis in sub-Saharan Africa. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 61(Suppl 5), S387–S388, https://doi.org/10.1093/cid/civ490
Shulman, J., Ahsan, R., & O’Malley, K. (2021). Understanding global vaccine economics and research and development. Powering Vaccine R&D: Opportunities for Transformation. Sabin-Aspen Vaccine Science & Policy Group.
United Nations. (n.d.). Transforming our world: The 2030 Agenda for Sustainable Development. Retrieved March 23, 2021, from https://sdgs.un.org/2030agenda
U.S. Department of Health and Human Services. (2021, March 2). Biden administration announces historic manufacturing collaboration between Merck and Johnson & Johnson to expand production of COVID-19 vaccines [Press release]. https://www.hhs.gov/about/news/2021/03/02/biden-administration-announces-historic-manufacturing-collaboration-between-merck-johnson-johnson-expand-production-covid-19-vaccines.html
U.S. Department of Justice. (2020, March). Joint antitrust statement regarding COVID-19. https://www.justice.gov/atr/joint-antitrust-statement-regarding-covid-19
U.S. Food and Drug Administration. (2017). 22 case studies where phase 2 and phase 3 trials had divergent results. U.S. Department of Health and Human Services. https://www.fda.gov/media/102332/download
U.S. Food and Drug Administration. (2020, December 14). Vaccine development—101. U.S. Department of Health and Human Services. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/vaccine-development-101
U.S. Food and Drug Administration. (2021, March 24). Emergency Use Authorization. U.S. Department of Health and Human Services. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
Watson, M. (2020, November 8). Personal correspondence with Vaccine Science & Policy Group staff.Watson, M., & Faron de Goer, E. (2016). Are good intentions putting the vaccination ecosystem at risk? Human Vaccines & Immunotherapeutics, 12(9), 2469–2474. https://doi.org/10.1080/21645515.2016.1172162
Weintraub, J. (2020, November 9). Personal correspondence with Vaccine Science & Policy Group staff.
Wilsdon , T., Lawlor, R., Li, L., Rafila, A., & García Rojas, A. (2020). The impact of vaccine procurement methods on public health in selected European countries. Expert Review of Vaccines, 19(2), 123–132. https://doi.org/10.1080/14760584.2020.1717952
Wolf, J., Bruno, S., Eichberg, M., Jannat, R., Rudo, S., VanRheenen, S., & Coller, B.-A. (2020). Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. npj Vaccines, 5(51), https://doi.org/10.1038/s41541-020-0204-7
World Health Organization. (n.d.-a). The Access to COVID-19 Tools (ACT) Accelerator. Retrieved March 24, 2021, from https://www.who.int/initiatives/act-accelerator
World Health Organization. (n.d.-b). Emergency use listing. Retrieved from https://www.who.int/teams/regulation-prequalification/eul
World Health Organization. (n.d.-c). Essential medicines and health products: About WHO prequalification of vaccines. Retrieved March 24, 2021, from https://www.who.int/medicines/regulation/prequalification/prequal-vaccines/about/en/
World Health Organization. (n.d.-d). Vaccines and immunization. Retrieved from https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1
World Health Organization. (2020a). Immunization agenda 2030: A global strategy to leave no one behind. https://www.who.int/docs/default-source/immunization/strategy/ia2030/ia2030-document-en.pdf
World Health Organization. (2020b, July 15). Immunization coverage. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
World Health Organization. (2020c, December 9). The top 10 causes of death. Retrieved from https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
World Health Organization. (2020d, December 18). COVAX announces additional deals to access promising COVID-19 vaccine candidates; plans global rollout starting Q1 2021 [Press release]. https://www.who.int/news/item/18-12-2020-covax-announces-additional-deals-to-access-promising-covid-19-vaccine-candidates-plans-global-rollout-starting-q1-2021
World Health Organization. (2021a, February 15). WHO lists two additional COVID-19 vaccines for emergency use and COVAX roll-out. https://www.who.int/news/item/15-02-2021-who-lists-two-additional-covid-19-vaccines-for-emergency-use-and-covax-roll-out
World Health Organization. (2021b, February 22). No-fault compensation programmed for COVID-19 vaccines is a world first. https://www.who.int/news/item/22-02-2021-no-fault-compensation-programme-for-covid-19-vaccines-is-a-world-first
Xue, Q. C., & Ouellete, L. L. (2020). Innovation policy and the market for vaccines. Journal of Law and the Biosciences, 7(1). https://doi.org/10.1093/jlb/lsaa026
Zimmer, C., Corum, J., & Wee, S.-L. (2021, March 25). Coronavirus vaccine tracker. New York Times. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html

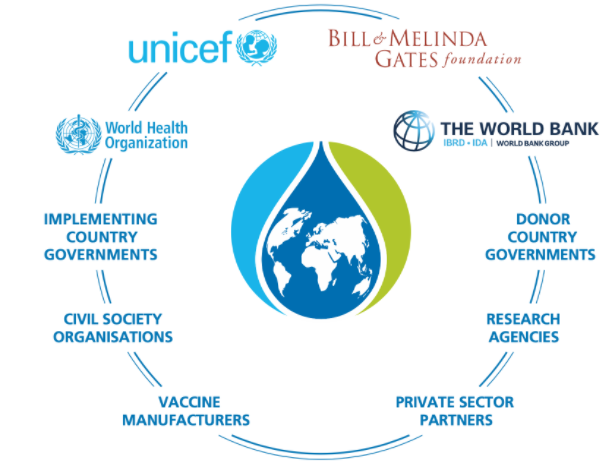
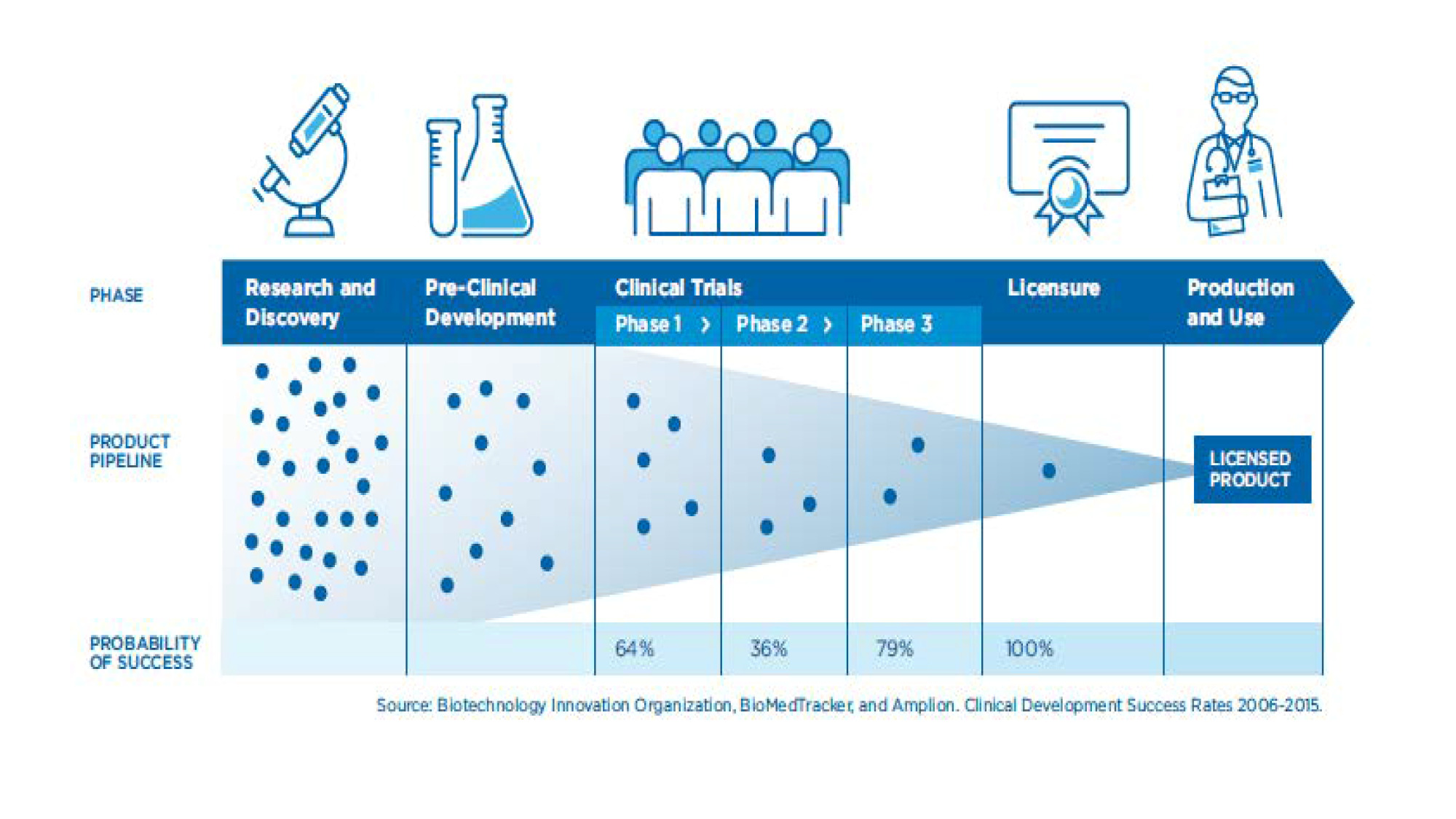
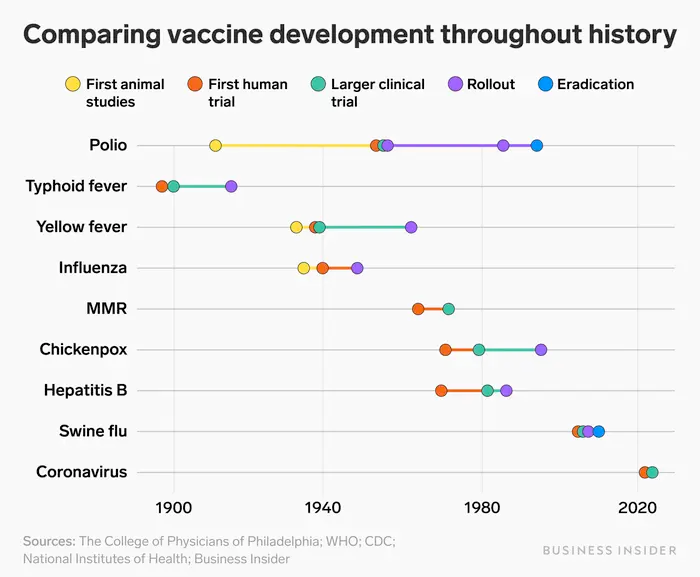
![Figure 3. COVID-19 vaccine clinical trials
Source: Romain Vuillemot – LIRIS, École Centrale de Lyon; Philippe Rivière – LIRIS, VisionsCarto; Pierre Ripoll – LIRIS, INSA Lyon; Julien Barnier – Centre Max Weber, CNRS. Retrieved from: https://covid-nma.com/vaccines/mapping/ [Online Resource]](/app/uploads/2021/04/Figure-3-graphic.png)